Recommended
The COVID-19 crisis has led to an unprecedented global response, including resource mobilisation to develop, manufacture, and distribute healthcare technologies. Protective equipment, diagnostic tests, oxygen and ventilators, drug treatments, and a vaccine against the virus, all form integral parts of an effective response to the pandemic and, once available, can be procured through dedicated structures set up by rich and poorer countries on a bilateral or collaborative basis. For low- and middle-income countries (LMICs) in particular, the World Bank; the Access to COVID-19 Tools Accelerator (ACT-A), cofounded by the WHO, World Bank, Global Fund for AIDS, Tuberculosis, and Malaria (GFATM), Gavi, the Vaccine Alliance, the Bill and Melinda Gates Foundation (the Gates Foundation) and Wellcome Trust, amongst others; and, more recently Africa Centers for Disease Control (CDC) PACT have all mobilised to help countries access the urgently needed COVID-19 technologies. Through these (and domestic channels), tens of billions of US dollars in grants, loans, and direct investment are being committed to procuring COVID-19 commodities in rich and poor countries alike.
But whilst agility and speed matter, the way these resources are (or are proposed to be) used—which technology, for whom, and at what price—are not being adequately addressed through due process and an end-to-end access framework (WHO published a basic outline for vaccine allocation last week).
With a focus on tests, treatments, and a vaccine, we take a look at the emerging global clinical and economic evidence-base underpinning some of these technologies, the mechanisms (mostly global) for financing these commodities, and, finally, the decision-making processes for selecting technologies. This includes identifying the right subpopulations and negotiating a cost-effective tiered price across countries and regions. We acknowledge the complexity of deploying technologies within clinical pathways and even broader non-pharmacological interventions; here we reflect on a few medical technologies and ask, “do they work, for whom, and what price is worth paying?”
The tale of two treatments
Intensive work is underway to investigate how a wide range of mostly existing medications can be “repurposed” to tackle COVID-19. Specifically, there are two which have already been approved for use in clinical practice and which have captured media attention. Remdesivir (an antiviral produced by Gilead) is one of the first COVID-19 treatments to be issued an emergency authorisation by the US FDA, conditional authorisation by the UK’s MHRA and the first COVID-19 treatment to receive EMA authorisation in late June 2020, for use in severely ill patients. With limited evidence of effect (the seminal NIH trial has shown no significant mortality benefit and further trials in less severely ill patients have shown marginal clinical benefit using a short as opposed to the longer course schedule), the drug has been distributed widely across the US and other high-income countries (HICs) on a first-come-first-served basis, undermining any prospect of understanding whether it actually works and for whom.
Given its widespread use in HIC intensive care units (ICUs), there have been some discussions as to an appropriate price, mainly in the US and surprisingly not in the UK, despite the latter’s tradition in cost-effectiveness analysis through NICE. The economic evidence considered so far has been as muddled as the clinical analyses. US ICER’s original options included a minimum marginal cost plus price (at $10 per dose) as well as an overly generous (given the lack of evidence of significant clinical benefit) value-based price (at $4,460 per dose). Discussions amongst investors have been equally inconclusive though there has been quite a bit of industry hype and a positive stock market response (Gilead’s stock is almost 20 percent up year-to-date). Elsewhere, the announcement of Gilead’s voluntary licensing arrangements (which allowed generic manufacturing to start in a number of countries, including Egypt but excluded Latin American countries) caused consternation amongst advocates pushing for access first and asking questions about effectiveness later (if at all), whilst the prospect of regulatory obstacles potentially delaying access contributed to the announcement of a wholesale reform of India’s regulatory process by the prime minister himself.
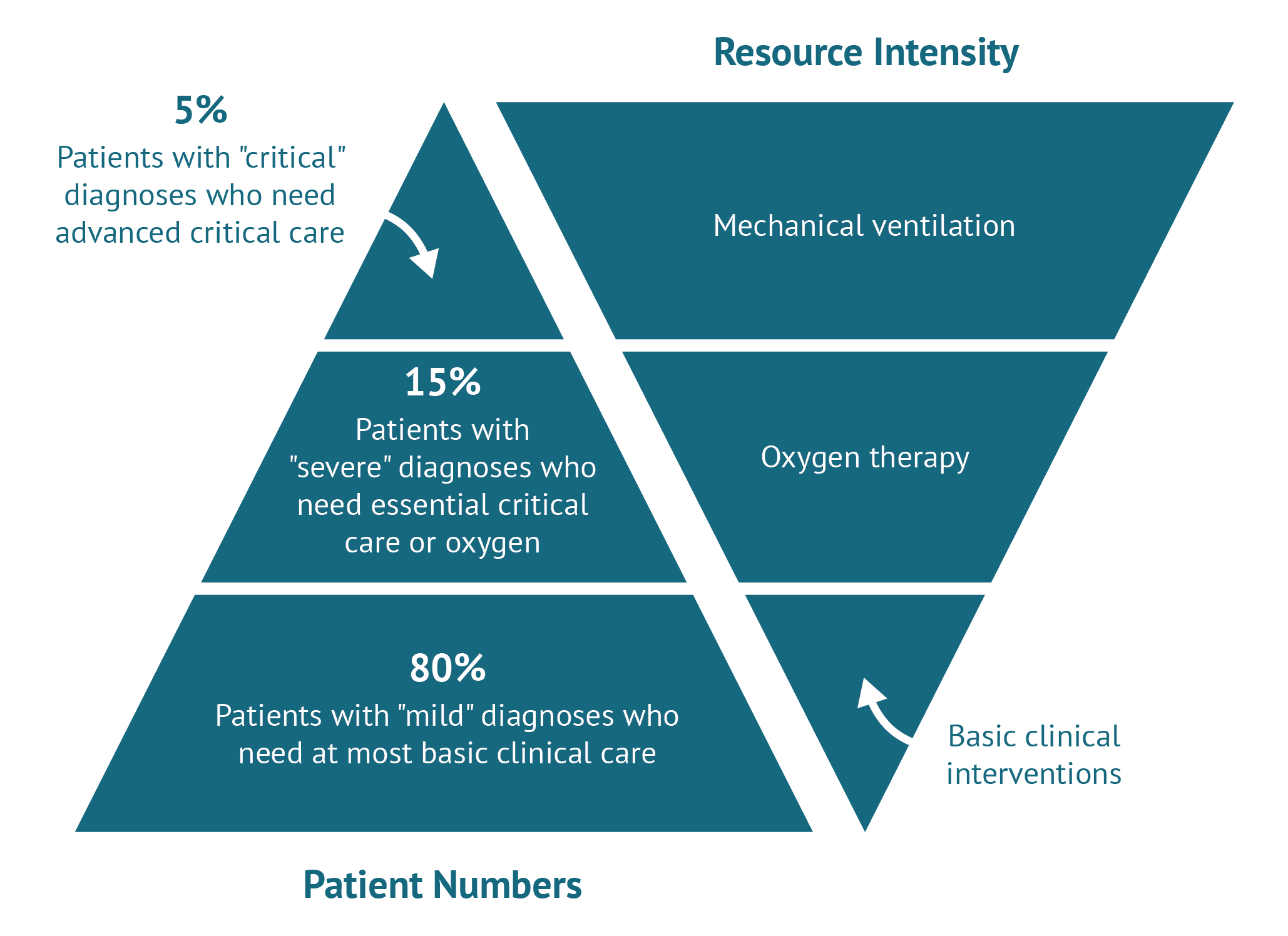
A few weeks later, a British group at the University of Oxford announced impressive results for an off patent, inexpensive and widely used drug, dexamethasone, among in-patients with COVID-19. The trial has yet to be subject to peer-reviewed publication but preliminary results show an impressive 35 percent mortality reduction for those on mechanical ventilation (most usually found in HIC ICUs). Those on oxygen but not mechanical ventilation also seem to be doing better (20 percent mortality reduction) compared to standard care, but it is unclear whether the supportive care they received in UK hospitals could be reproduced in LMIC settings. Past experience of extrapolating to LMICs suggests caution, and a large multi-centre multi-country pragmatic randomized control trial (RCT) in LMICs should be undertaken (WHO’s Solidarity trial includes Remdesivir but not dexamethasone) before definitive guidance is issued given potential side effects and weaker infrastructure (e.g. limited access to ventilation or high flow oxygen which seemed to have been crucial in realising the benefits dexamethasone). The UK announced this was to become the standard of care and simultaneously moved to ban it from exports (the drug was reportedly already in short supply in LMICs even before the outbreak and, as of 26 June 2020, is listed as currently in shortage by the FDA). NICE, in the meantime, issued an evidence summary, outside its traditional HTA process, which notes the lack of any evidence on cost effectiveness. No economic modelling study seems to have been commissioned and no clear recommendation is offered to the NHS as to remdesivir’s use or, most importantly, price (we could not find remdesivir listed in the British National Formulary either).
Shortly after, ICER revised downward its value-based price for remdesivir to $2,800 per dose. That is still on the high end given there is still no proof of a survival benefit (indeed ICER’s value-based price based on what the trials have shown conclusively so far is $310 assuming a $50,000 per QALY threshold—on the high end for most HICs let alone LMICs). On the bright side for Gilead, ICER also revised upwards the drug’s cost plus price to over $1,000 citing Gilead information on R&D investment. As this blog was going to press, Gilead announced a $2,340 price tag for a five-day course for the US public market and a higher price for private insurance. Assuming trials show the drug reduces mortality, this may be an example of responsible pricing. The same logic suggests that if trials show no benefit, then the company will drop its price.
Test, test, test!
Testing for SARS-CoV-2 virus has been the centrepiece of WHO’s global advice since the early days of the outbreak. More recently antibody testing has also been added. But devising and rolling out an adaptable dynamic testing strategy that adds value to public health interventions and/or clinical care is no mean feat. Different types of test need to come together to address different but complementary questions at different points of the outbreak, all whilst the epidemiology and our understanding of the biology of the virus and our immune response to it are constantly changing. If testing strategies are not carefully designed, and efficiently implemented, they may even slow down syndromic-based contact tracing methods, exacerbating the epidemic.
Here again the evidence of what works for whom and what the right price point may be, is weak. After months of trying to stand up an operational test treat isolate (TTI) framework, the UK government announced through accelerated and private proceedings the approval of two antibody tests, initially by Roche and then Abbott. The secretive process of selecting and evaluating the winners triggered concerns, amplified more recently with the government’s announcement to scale up testing across the country. Lack of a strategy (or clinical indications), lack of a performance standard against which to verify testing accuracy, and lack of transparency on the opportunity costs of such a nationwide effort in terms of money and GPs time were all cited as concerns, as were perceived conflicts amongst those advising the government. All of the above could have been addressed (if not resolved) through a NICE process of approval, but again, there is no public reference to NICE nor a NICE guidance on testing (NICE has launched an “exploratory” economic modelling project for point of care COVID-19 diagnostics due to report in February 2021). Confusion about a testing strategy is present throughout the world: in India, where national scale up capacity and coordination is a challenge, and in South Africa, where very slow turnaround times despite plenty of testing capacity made the country’s initially praised TTI approach unsustainable and ineffective. Kenya, which initially indicated a plan to do mass testing despite clear capacity challenges, in the end implemented targeted testing of high-risk groups and symptomatic individuals. And Ghana, originally praised for its “targeted mass testing” approach, has been forced to scale down significantly due to mainly resource constraints. The lack of stratified population testing strategies in most countries continues to mean they are flying blind, with little understanding of their stage in the epidemic.
WHO has issued an overwhelming volume of guidance including on serology, laboratory and supply chains, but we have not been able to identify specific guidance on national testing strategies by epidemic stage and country infrastructure and resources. (The search for testing returns several documents).
A vaccine: The definitive solution(?)
The vaccine situation is no better, as any evidence to inform adoption decisions is not yet available. Instead and under pressure to act, individual HICs such as the UK, Germany and France, and the US, and HIC clubs such as the EU have come together to commit to buy at the early development stage with no population efficacy data. Gavi, under the ACT Accelerator, is working on its own agreement with low- and lower-middle-income countries in mind. Upper-middle-income countries (UMICs) seem to be largely left out up until now.
One common feature of these advanced purchase deals is they lack transparency (also here). Another is that they do not consider evidence (even conditional) of what works in their design. As a result, the dynamic effects of these advance purchasing decisions may well undermine the result of the vaccine race, potentially deterring companies who are behind the frontrunners from completing their R&D on the presumption that there will be no demand left by the time they get to market. This is the last thing we want to encourage, especially as the changing epidemiological profile and immune response at population level enhance the uncertainty surrounding such early bets. These criticisms are not new: they were levelled against another advance purchase commitment led by Gavi several years ago, aimed at bringing to market a PCV vaccine.
At the same time there are global (WHO-led) and domestic (e.g. UK Joint Committee on Vaccination and Immunisation) efforts to set out allocation rules, though given the uncertainty regarding the epidemiological realities several months, perhaps years, into the future and vaccine performance (which is not part of the ex ante deals), these are limited to general principles on prioritising based on clinical need.
How do we pay?
Tens of billions of US dollars have been committed to commodity procurement, a large chunk of this to tests, treatments, and for the development and manufacture of a vaccine (although it is not easy to find exactly how much and for what). Just days ago the GFATM called for another $5 billion to invest in the COVID-19 response and in protecting essential AIDS, tuberculosis, and malaria services. The ask for the next 12 months for GFATM eligible countries to protect the gains in the three diseases and also fight the virus stands at over $28 billion, with almost $5 billion on diagnostics and another $8 billion treatments, all in addition to what has already been committed. A total ask of $31.3 billion (which includes the $28 billion ask set out by the GFATM) was announced soon after by the WHO ACTA (which includes GFATM) covering vaccines, treatments, and diagnostics, from R&D to manufacturing and distribution over then next 12-18 months. In parallel, African CDC and the World Bank will continue to play a central and emerging (in the case of CDC) role in commodity procurement as will national treasuries in HICs and LMICs alike.
But, decisions to allocate mobilised resources at country level have oftentimes been rushed and non-transparent, leading to purchases perhaps not obviously useful or useable given the local infrastructure and human resources, as is the case for Kenya. With a prolonged global recession highly likely, the opportunity costs of inefficient allocation are going to be felt for years to come. Cost-effectiveness has explicitly featured in none of these discussions, whether in the context of global buyers such as WHO ACTA and World Bank or indeed in the case of countries such as the UK with a tradition in evidence-based medicine and health technology assessment (HTA). Opportunities for pooled procurement based on credible demand forecasting and linked to local context and affordability have yet to be taken up. A state of “emergency response” combined perhaps with the laudable but potentially inequitable principle of putting lives first and considering economic costs later (if at all) may help explain the lack of due process and a reluctance to consider all the evidence. But the magnitude and prolonged duration of the crisis underlines the need for a more (not less) holistic and dynamic consideration of uncertainty and new evidence in the context of an open consultative and insulated-from-interests process.
We need a process to build trust and enhance our chances of making the right (and impactful) decisions
There is still time to wrap a process around the billions of US dollars channelled towards healthcare commodities domestically and globally and in doing so make it more likely that better decisions are made, improving the odds of beating COVID-19 sooner (and for less money). A process which intrinsically (through building trust amongst the scientific community and public opinion, tackling vaccine hesitancy for one) and instrumentally (through crowding in better information and undergoing peer review) leads to better decisions because it:
-
acknowledges uncertainty (and rejects incredible certitude) and both uses existing and encourages the generation of new evidence of what works as technologies are understandably rolled out in a fast tracked fashion;
-
allows for conflicts of interest of individuals and stakeholders to be declared and managed instead of the real (or perceived) undue influence by commercial and academic interests undermining the credibility of the decisions;
-
applies the principles of evidence-based medicine and health technology assessment to the evaluation of health technologies, current and future ones;
-
engages stakeholders in a deliberative process to consider trade-offs with other health priorities and the needs of different population sub-groups and to build trust in its outputs.
The COVID-19 pandemic is far from over. There is still an urgent need to develop and deploy policies and individual technologies for managing its multiple impacts. This is the time for global (GFATM, WHO) and national (such as the UK) payers to revert to (instead of undermining) known and tested mechanisms such as Health Technology Assessment as carried out by NICE (domestically and internationally applied) for assessing comparative clinical and cost effectiveness of medical technologies placing their faith on evidence, value for money, and due process. This is the right and the smart thing to do.
Disclaimer
CGD blog posts reflect the views of the authors, drawing on prior research and experience in their areas of expertise. CGD is a nonpartisan, independent organization and does not take institutional positions.