Recommended
Introduction
Over the past two decades, partnerships between bilateral and multilateral funders, philanthropy, national governments, and the private sector have substantially increased global access to effective malaria treatment. Nevertheless, further action on malaria case management is needed to meet global targets for reduced malaria morbidity and mortality.
The quality and affordability of malaria case management within private sector drugstores, clinics, and pharmacies—where 30 percent[1] of febrile illnesses are treated (WHO, 2020)—requires particular attention. Despite prior interventions, serious problems remain, including poor diagnostic practices and incentives for overtreatment. New technology solutions and data can help understand local context and design tailored country-specific strategies for intervention. Such solutions must consider and respond to the incentives of all relevant actors (e.g., drug regulatory agencies, manufacturers, retail pharmacists, physicians, patients/caregivers); they also need to be contextually appropriate given the funding environment for malaria interventions, broader efforts to improve health system performance, ambitions for universal health coverage (UHC), and the global fight against anti-microbial resistance.
This note draws on key informant interviews, discussions at a 2-day meeting of key stakeholders, and a desk review of grey and published literature to generate initial recommendations on improving malaria case management in the private sector. Key informant interviews were conducted with academics, industry representatives, government officials, and foundation staff, among others. The full set of interviewees is listed in Appendix A in the PDF version of this note. Several interviewees also attended in the stakeholder convening that occurred on February 4 and February 5, 2021. The full set of attendees at the stakeholder meeting is listed in Appendix B in the PDF version of this note.
Background
The private sector is a major source of treatment for malaria (and febrile illnesses more generally) in malaria-endemic countries. While the role of the private sector varies from one country to another, approximately 35 percent of all patients with suspected malaria globally seek care in the private sector. The private sector is highly diverse in its form and size; private sector providers include everything from large hospitals to well-staffed pharmacies, smaller drug stores, and sometimes even street vendors. Drug stores were the predominant outlet stocking and selling antimalarials in the DRC. Nigeria, Uganda, and Zambia but in Benin and Madagascar, general stores were the predominant outlet for antimalarials rials, by outlet type (O’Connell et al., 2011).
The private sector has long been recognized as a significant provider of malaria treatments, but concerns about provider quality, substandard medicines, and presumptive diagnosis have limited the engagement of global agencies and the use of development assistance for health (DAH) to improve case management in the private sector. Most private pharmacies and drug shops still practice presumptive diagnosis of malaria even in contexts where doing so is clinically inappropriate; the availability of rapid diagnostic tests (RDTs) or malaria microscopy among private-sector anti-malarial stocking outlets in 2015 or 2016 was less than 40 percent in eight sub-Saharan African countries (PSI & ACTwatch, 2017). The diversity of the private sector for malaria case management complicates efforts at regulation and quality control, while also making it difficult for patients to identify high-quality providers. Finally, the quality of antimalarial drugs available in the private sector continues to be an area of concern.
Prior efforts to address the challenge
The Affordable Medicines Facility-malaria (AMFm) offered a factory-gate subsidy for pre-qualified artemisinin-based combination therapies (ACTs) in seven countries with the aim of increasing the affordability, availability, and use of ACTs and decreasing the use of artemisinin monotherapies and chloroquine. The program ran from 2011 through 2016 before it was discontinued in 2017 as a separate financing mechanism. During its period of operation, the AMFm successfully decreased ACT retail prices, increased the availability of ACTs in the private sector, and decreased the use of non-artemisinin-based combination therapy (Rosen et al., 2020; Tougher et al., 2017). Key informant interviews revealed that in some countries the manufacturer level subsidy on ACTs was not adequately passed on to the consumer; there was also significant subnational variation in prices, particularly in areas far from big towns that proved difficult to monitor. While there were concerns at the start of the AMFm pilot that the reach and price pass through of the subsidy will be lower in rural and remote areas, operational research carried out under AMFm revealed that was not the case (Yadav et al., 2012; Ye et al., 2015). The AMFm subsidy only applied to ACTs and did not include any systematic efforts to incentivize the use of rapid diagnostic tests. The AMFm subsidized only WHO prequalified (PQ) manufacturers of ACTs; local manufacturers in participating countries were thus ineligible for the subsidy, though national governments continually stressed the need to include local manufacturers or create specific programs to help them become eligible.
The Global Fund’s Private Sector Co-Payment Mechanism, the successor to the AMFm was built on the same operational model as the AMFm i.e., providing a co-payment from the Global Fund to manufacturers with Global Fund-negotiated prices (GFATM, 2021). The difference was that unlike the AMFm pilot where copayment was provided through a separate pool of money, under this new model eligible countries allocated funding toward this mechanism from their core Global Fund grant allocations (GFATM, 2012), effectively delegating decisions on the level of subsidy and intervention design to country managers. In this context, most countries either ended or dramatically decreased their subsidies due to competing budget priorities (WHO, 2019). The reduction and/or discontinuation of the ACT subsidy is now visible in import prices, where the cost of quality-assured ACTs now exceeds average import costs for ACTs that have not received WHO prequalification (WHO, 2019).
To address the gap in appropriate diagnosis, Unitaid’s 2013-2016 project on private sector RDTs aimed to increase the availability of RDTs in Kenya, Madagascar, Nigeria, Tanzania, and Uganda through subsidies for RDTs, consumer marketing efforts, training providers, and advocacy for regulatory change (Dalberg, 2016). While the project faced various logistical, budget, and oversight challenges, it did successfully increase demand for quality assured RDTs, though demand levels were lower than expected in three countries and may have been concentrated among wealthier customers.
The current state of private sector case management: Progress and remaining challenges
At the conclusion of the AMFm, policymakers feared that improvements in quality ACT access would erode without continued subsidies; higher ACTs prices would then lead patients to switch back to inferior monotherapies. This worst-case scenario has not come to pass; recent data suggests that in the 7 countries in SSA where AMFm was piloted, the shift from monotherapies to ACTs has endured for a decade after the AMFm concluded (WHO, 2019). Nevertheless, there are several ongoing challenges in private sector malaria case management.
Challenge 1: ACTs of unknown quality
Without the subsidy intervention, we see a substantial market shift away from WHO prequalified products to products of unknown quality (Figure 1) (WHO, 2019). Non-prequalified ACTs are not necessarily of lower quality, but they are of unknown quality—which means, definitionally, that they are at higher risk of being substandard than those that receive WHO prequalification. Substandard drugs pose safety risks and may drive further drug resistance, with significant consequences for health outcomes and the economic costs faced by countries (Ozawa et al., 2019). Previous analyses across Sub-Saharan Africa have found that substandard and falsified antimalarials contribute to between 3.8 and 8.9 percent of malaria deaths (WHO, 2017). The Substandard and Falsified Antimalarial Research Impact (SAFARI) model has been applied to estimate the health and economic impact of substandard and falsified antimalarials among children under five in four countries and has shown significant impact on both pediatric and adult malaria burden (Beargie et al., 2019).
Figure 1. Market share of ACTs by WHO prequalification status in Kenya, 2017-2020
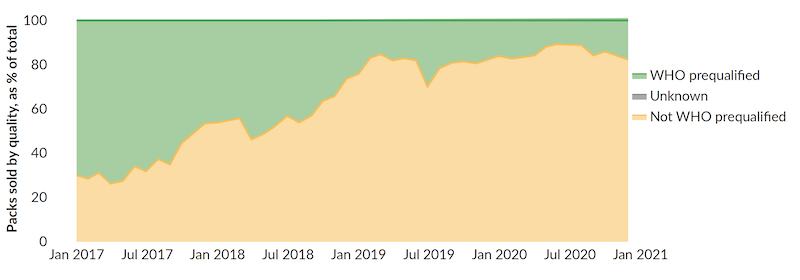
Source: Maisha MedsChallenge 2: Continued presumptive diagnosis
Source: Maisha Meds
Presumptive treatment of febrile illness is no longer justified in most settings, yet most pharmacists still do not routinely use RDTs prior to offering ACT. Offering ACT without RDTs is common even in countries where doing so runs contrary to the National Malaria Control Program’s official policy (Bonful et al., 2019). Pharmacists have little to no incentive to deviate from the presumptive treatment of febrile illness. Focus group discussions on inappropriate prescribing practices have identified pressure from patients, a desire to avoid losing their patients to another provider, diagnostic uncertainty, poor teamwork, and influence from medical representatives as underlying reasons for such behavior (Ncube et al., 2020). Similarly, patients have limited incentives to pay for a diagnosis. Many countries also have regulatory restrictions that prevent pharmacists from administering RDTs but allow ACT prescriptions (Maloney et al., 2017). The persistence of the presumptive diagnosis approach has two main consequences. First, the overuse of ACTs may contribute to drug resistance. Second, people with misdiagnosed febrile illness will not receive appropriate treatment. This has a disproportionate impact on the poor.
However, it is important to note that presumptive diagnosis is not only a problem in the private sector. While diagnosis in public sector clinics has improved, it is still high in many countries. The overall (public + private sector) rate of malaria diagnosis using RDTs or microscopy among children under 5 years is still less than 38 percent, suggesting that even in public sector clinics many children are dispended ACTs without parasitological diagnosis (WHO, 2020).
Challenge 3: Siloed vs. systems approach
A siloed approach to malaria case management can leave people suffering from febrile illness—but with a negative RDT result for malaria—without a clear path forward to address their health concerns. Well-designed and well-resourced referral systems are critical to ensure patients with other non-malarial febrile illnesses receive appropriate care. Such referral systems need adequate tools for risk triaging so that non malarial febrile illnesses which have early signs of illnesses which are not likely to be self-resolving and would require attention at a health facility are referred. Nonetheless, formal referral systems between private providers and other facilities are not always in place (Visser et al., 2017). In addition, numerous supply- and demand-side issues limit the efficacy of existing systems. For example, providers who infrequently referred patients with negative RDT results raised concerns about potentially weakening their own client base through referrals (Visser et al., 2017). Patients and their families may also fail to follow up on referrals due to resource constraints and gender imbalances in decision-making power, as well as skepticism about the quality of care at referral facilities (Mbonye et al., 2017).
Integrated Community Case Management iCCM offers an alternative approach with comprehensive diagnosis and treatment of pneumonia, diarrhea, and malaria through community health workers or health extension workers. iCCM programs also provide a platform for facilitating referral of severe illness. However, large funding streams are not available for iCCM approaches; most available resources are focused on malaria interventions alone. In addition, iCCM adds more complexity to the training modules for private retailers and drug shops as they have to be trained to manage across multiple diseases. The sheer number of private sector outlets is so large that carrying out such training using conventional approaches comes with significant costs and overheads.
Many of the needed reforms to improve malaria case management require intervention at a higher level and more fungible funding—for example, regulatory reform for informal drug-sellers; pharmacovigilance; and improvements to the accreditation system. Some existing initiatives for prevention, surveillance, and diagnosis of antimicrobial resistance (AMR) require similar system reforms to those needed for malaria treatment, suggesting some untapped opportunities for integration and collaboration.
Challenge 4: ACT and RDT financing constraints
Financing for malaria treatment is heavily dependent on development assistance for health (DAH). In 2016, the $2.4 billion in DAH for malaria represented more than half (56.5 percent) of total malaria spending.
Theoretically, monies from DAH dedicated to malaria are available to support case management in the private sector, but practical constraints prevent any significant outlays to private sector treatment access. The Global Fund’s financing is “demand-driven,” with grants focused on interventions and areas of support highlighted by Country Coordination Mechanisms and in turn by NMCPs. A significant portion of available funds is allocated to preventive interventions such as ITN, IRS, IPTp, leaving funding for ACT and RDTs highly constrained even for the public sector. In this highly resource-constrained context, the private sector becomes an afterthought for most NMCPs; very few countries highlight private sector case management as a priority area within their Global Fund grants or PMI Malaria Operational Plans. There are a few designated pockets of money which can be used to improve private sector treatment, but they are few and relatively small.
The challenges are likely to become more entrenched as malaria endemic countries grow economically and lose eligibility for malaria DAH. The long-term sustainability of malaria programs will depend on a country’s ability to continue financing malaria interventions without financial support from the Global Fund and PMI. However, few countries have clear plans to raise domestic financing for their NMCPs. In such an environment, and given competing demands both within malaria and across the overall health sector, few countries are likely to adequately fund private sector strategies to improve malaria case management.
Challenge 5: Data availability and quality
Numerous factors undermine private sector providers’ incentives to collect and share high-quality data on the malaria cases they see. For example, private sector providers do not receive commodities or diagnostics from the national malaria control program and enforcement of data reporting policies are often applied inconsistently (Githinji et al., 2017). As a result, reporting rates among private sector facilities to key databases, such as DHIS2, are quite low (Bennett et al., 2017). High quality survey data on antimalarial and diagnostic markets that can be used for cross-country comparisons is also limited following the end of ACTwatch in 2017 (ACTwatch, 2017). ACTwatch served as the most reliable source of nationally representative data on the availability, price, and market share of antimalarial medicines and diagnostics in the public and private sector.
Opportunities
Despite these challenges, we identified several opportunities:
Opportunity 1: Tiered accreditation
Tiered accreditation is an approach to gradually improve quality standards among drug-sellers and bring informal dispensers in line with national regulations. Tiered accreditation is more than a branding approach; it is tied to changes in the regulatory regime and the reimbursement policies of insurers. These changes create important incentives for drug-sellers to become accredited. In Tanzania, accredited drug dispensing outlets (ADDOs) are allowed to sell certain prescription-only medicines. Drug shops also have numerous additional incentives to participate in the ADDO program, such as access to business management training, microfinancing, and an ADDO-specific pharmaceutical wholesalers (Edmund Rutta et al., 2009).
Opportunity 2: Digital tools and new data
Digital tools present new opportunities to gather routine, near real-time data on market activity and trends. Digital tools are improving data quality and use of data for decision-making in LLIN distribution, ACT stock monitoring, and a variety of other malaria activities. Electronic point of sales (e-POS) systems in public and private health facilities enable tracking of treatment dispensed. Cell-phone cameras enable the use of pictures or QR codes to document RDT results and support third-party reimbursement. Mobile money and mobile health wallets can also facilitate reimbursement or targeted subsidies from a payer at the point-of-sale (Smith et al., 2019). New digital solutions on stock and flow monitoring, price monitoring, end use verification, and RDT use verification can enable a new paradigm in malaria case management in the private sector. Digital training tools can also reduce the cost of training private providers on malaria case management, iCCM and risk-based referral algorithms.
Opportunity 3: Integration with UHC agenda
Universal health coverage (UHC) is rapidly emerging as an essential framework under which health financing and service delivery is organized. As countries define their UHC structure, it is important to ensure that cost effective malaria interventions are embedded into UHC packages. Malaria treatment and diagnosis are highly cost-effective and should be included in health benefits packages. Including malaria treatment and diagnosis in health benefits packages could create a potentially sustainable source of financing for getting private providers “in network” reimbursement for both services. Eligibility for the UHC “network” could be tied to accreditation level, registration with a regulatory agency, or adherence to quality standards as measured via surveys or mystery shopper visits.
Opportunity 4: Regional manufacturing
National policymakers see domestic or local manufacturing as a priority, especially in the aftermath of COVID-19 supply chain interruptions. However, such manufacturing has historically been less of a priority for donors and the international community. The momentum from increased regional purchasing, cooperation, and regulatory harmonization efforts, such as the African Union’s purchase agreements for COVID-19 vaccines, could provide a boost to regional manufacturing efforts and ultimately help increase access to quality ACTs.
Here, a key challenge is persuading local manufacturers to pursue pre-qualification status. Leveraging sources of financing such as the International Finance Corporation and the US Development Finance Corporation could help countries develop regional manufacturing hubs and create incentives for quality improvement.
Recommendations
Achieving the WHO’s Global Technical Strategy (GTS) targets for malaria—a reduction of at least 90 percent in malaria case incidence and mortality rates, and elimination in at least 35 countries by 2030—will require increasing the proportion of malaria cases which receive prompt access to diagnosis and treatment. Given that a significant proportion of those seeking treatment in public sector facilities already receive treatment with ACTs—and given that an estimated 30 percent of patients with malaria-like fevers who received treatment were treated in private sector facilities (WHO, 2020)—a serious effort to reach GTS diagnosis and treatment targets cannot simply ignore private sector case management.
As briefly outlined above, there are real opportunities to address case management in the private sector through a series of short term and medium-term solutions. Doing so will require locally tailored solutions for the health-system context of each country, informed by knowledgeable local experts. However, an overall framework for financing, evidence generation, and adaption/creation of fit-for-purpose digital tools should be supported by global donors and technical agencies. Below, we lay out three main recommendations for global action.
Recommendation 1: Update the evidence on cost-effectiveness for private sector malaria case management recognizing new digital tools for targeting.
Prevention and effective treatment of malaria is highly cost effective and can result in large health gains. However, there is limited up to date and robust evidence on the cost effectiveness of interventions to increase access to diagnosis and treatment in the private sector. There are multiple small sample studies, but there is considerable variation in the assumptions, scale, and results across these studies.
In the absence of robust evidence on the cost effectiveness of treatment in the private sector in terms of health outcomes, i.e., disability-adjusted life years (DALYs) averted, private sector case management is not perceived to rank among the best "value for money" interventions. Prudent decision-makers are thus likely to select alternative malaria (or non-malaria) interventions to receive scarce funds. Yet new practices for private sector intervention and precise targeting—enabled by digital technologies in the private retail sector—may prove highly cost effective if appropriately evaluated.
PMI, the Global Fund, the Bill & Melinda Gates Foundation and UNITAID should finance a research program to generate and synthesize updated evidence on the cost-effectiveness of targeted interventions to manage malaria in the private sector. The research program should build on ongoing efforts to measure the impact of targeted subsidies and focus on the areas which have the greatest parameter uncertainty in current modelling-based estimates of cost effectiveness. In turn, a more robust understanding of these interventions’ cost-effectiveness is likely to stimulate greater interest and funding, both at the domestic and international levels.
Recommendation 2: Carry out a techno-economic feasibility study of targeted retail subsidies for ACTs and RDTs in high burden malaria countries in Africa
Conventional approaches to enhancing affordability of ACTs and RDTs have involved a top-level subsidy, either at the manufacturer factory gate or at the national wholesaler/distributor level. The transaction costs of targeted retail level subsidies were assumed to be too high to make them cost effective. The solution to this issue lies in utilizing new digital technologies for targeting and end user verification.
Such solutions appear feasible in small pilots but have not yet been demonstrated at scale. The Bill & Melinda Gates Foundation, in partnership with UNITAID, PMI and the Global Fund, should fund a technological and economic feasibility study of a large scale digitally targeted subsidy for ACTs and RDTs at the retail and/or end-patient level. Such a study would synthesize evidence from multiple smaller scale pilots, including a recent project funded by PMI. In addition to helping clarify the technical requirements for such an endeavor, the project would inform cost estimates and generate data about current treatment seeking behavior.
Recommendation 3: Establish a working group to address cross-cutting issues in private sector case management.
The Global Fund, PMI, UNITAID, WHO-GMP and other partners should establish an ad-hoc working group to address a range of cross cutting issues such as regulation, pricing, and financing. This would serve as a platform to develop a common roadmap for investments in private sector case management. It would also act as a group which would discuss and disseminate the results of the cost effectiveness and techno-economic feasibility studies proposed above. The group would work to proffer a more robust standard for “quality” in management of acute febrile illness, with attention to differences between patient perception of quality differs and the provider/public health perspectives
In addition, this group would serve as a bridge between different system-level areas which are important to include in designing a sustainable intervention for effective case management in the private sector. In particular, it would liaise with:
- Groups working on pathways for pharmacy/retail-based HIV Testing and COVID diagnostics, to understand commonalities in behavioral factors, economic incentives, and referral linkages factors associated with testing.
- Pharmacy/pharmacy practice regulation to allow RDT use in retail settings.
- ALMA-AU-NEPAD group working on regional manufacturing of medicines to ensure that ACTs and RDTs are included and prioritized for investment in regional manufacturing where possible.
- National policymakers and local and international groups working to establish and expand evidence based UHC, including the Joint Learning Network and UHC2030.
References
ACTwatch. (2017). ACTWatch - MAP. The Malaria Atlas Project. https://malariaatlas.org/actwatch/
Beargie, S. M., Higgins, C. R., Evans, D. R., Laing, S. K., Erimid, D., & Ozawaid, S. (2019). The economic impact of substandard and falsified antimalarial medications in Nigeria. PLoS ONE, 14(8). https://doi.org/10.1371/journal.pone.0217910
Bennett, A., Avanceña, A. L. V., Wegbreit, J., Cotter, C., Roberts, K., & Gosling, R. (2017). Engaging the private sector in malaria surveillance: A review of strategies and recommendations for elimination settings. In Malaria Journal (Vol. 16, Issue 1, p. 252). BioMed Central Ltd. https://doi.org/10.1186/s12936-017-1901-1
Bonful, H. A., Awua, A. K., Adjuik, M., Tsekpetse, D., Adanu, R. M. K., Nortey, P. A., Ankomah, A., & Koram, K. A. (2019). Extent of inappropriate prescription of artemisinin and anti-malarial injections to febrile outpatients, a cross-sectional analytic survey in the Greater Accra region, Ghana. Malaria Journal, 18(1). https://doi.org/10.1186/s12936-019-2967-8
Dalberg. (2016). Unitaid end of project evaluation: Creating a private sector market for quality-assured mRDTs Unitaid end of project evaluation: Creating a private sector market for quality-assured mRDTs-EXECUTIVE SUMMARY. http://www.unitaid.eu/en/amfm
Edmund Rutta, Katie Senauer, Keith Johnson, Grace Adeya, Romuald Mbwasi, Jafary Liana, Suleiman Kimatta, Margareth Sigonda, & Emmanuel Alphonce. (2009). Creating a New Class of Pharmaceutical Services Provider for Underserved Areas: The Tanzania Accredited Drug Dispensing Outlet Experience. Progress in Community Health Partnerships: Research, Education, and Action, 3(2), 145–153. https://doi.org/10.1353/cpr.0.0063
GFATM. (2012, November 15). Board Approves Integration of AMFm into Core Global Fund Grant Processes - News & Stories - The Global Fund to Fight AIDS, Tuberculosis and Malaria. The Global Fund. https://www.theglobalfund.org/en/news/2012-11-15-board-approves-integration-of-amfm-into-core-global-fund-grant-processes/
GFATM. (2021). Procurement Tools - Sourcing & Management of Health Products - The Global Fund to Fight AIDS, Tuberculosis and Malaria. The Global Fund. https://www.theglobalfund.org/en/sourcing-management/procurement-tools/
Githinji, S., Oyando, R., Malinga, J., Ejersa, W., Soti, D., Rono, J., Snow, R. W., Buff, A. M., & Noor, A. M. (2017). Completeness of malaria indicator data reporting via the District Health Information Software 2 in Kenya, 2011-2015. Malaria Journal, 16(1), 2011–2015. https://doi.org/10.1186/s12936-017-1973-y
Maloney, K., Ward, A., Krenz, B., Petty, N., Bryson, L., Dolkart, C., Visser, T., le Menach, A., Scott, V. K., Cohen, J. M., Mtumbuka, E., & Mkude, S. (2017). Expanding access to parasite-based malaria diagnosis through retail drug shops in Tanzania: evidence from a randomized trial and implications for treatment. Malaria Journal, 16(1), 1–10. https://doi.org/10.1186/s12936-016-1658-y
Mbonye, A. K., Buregyeya, E., Rutebemberwa, E., Clarke, S. E., Lal, S., Hansen, K. S., Magnussen, P., & LaRussa, P. (2017). Referral of children seeking care at private health facilities in Uganda. Malaria Journal, 16(1), 76. https://doi.org/10.1186/s12936-017-1723-1
Ncube, N. B. Q., Knight, L., Bradley, H. A., Schneider, H., & Laing, R. (2020). Health system actors’ perspectives of prescribing practices in public health facilities in Eswatini: A Qualitative Study. PLoS ONE, 15(7 July). https://doi.org/10.1371/journal.pone.0235513
O’Connell, K. A., Gatakaa, H., Poyer, S., Njogu, J., Evance, I., Munroe, E., Solomon, T., Goodman, C., Hanson, K., Zinsou, C., Akulayi, L., Raharinjatovo, J., Arogundade, E., Buyungo, P., Mpasela, F., Adjibabi, C. B., Agbango, J. A., Ramarosandratana, B. F., Coker, B., … Chavasse, D. (2011). Got ACTs? Availability, price, market share and provider knowledge of anti-malarial medicines in public and private sector outlets in six malaria-endemic countries. Malaria Journal 2011 10:1, 10(1), 1–14. https://doi.org/10.1186/1475-2875-10-326
Ozawa, S., Haynie, D. G., Bessias, S., Laing, S. K., Ngamasana, E. L., Yemeke, T. T., & Evans, D. R. (2019). Modeling the economic impact of substandard and falsified antimalarials in the Democratic Republic of the Congo. American Journal of Tropical Medicine and Hygiene, 100(5), 1149–1157. https://doi.org/10.4269/ajtmh.18-0334
PSI, & ACTwatch. (2017). Malaria market trends in Sub-Saharan:2009-2016. www.actwatch.info
Rosen, D., Vernon, J., Silverman, R., Juma, E., Dieci, M., & Yadav, P. (2020). Malaria Case Management After the Affordable Medicines Facility for Malaria (AMFm): Availability, Quality, and Market Share for ACTs in Kenya’s Private Pharmacies | Center For Global Development (No. 561). /publication/malaria-case-management-after-affordable-medicines-facility-malaria-amfm-availability
Smith, S., Koech, R., Nzorubara, D., Otieno, M., Wong, L., Bhat, G., van den Bogaart, E., Thuranira, M., Onchonga, D., & Rinke De Wit, T. F. (2019). Connected diagnostics: Linking digital rapid diagnostic tests and mobile health wallets to diagnose and treat brucellosis in Samburu, Kenya. BMC Medical Informatics and Decision Making, 19(1). https://doi.org/10.1186/s12911-019-0854-4
Tougher, S., Mann, A. G., Group, Act., Ye, Y., Kourgueni, I. A., Thomson, R., Amuasi, J. H., Ren, R., Willey, B. A., Ansong, D., Bruxvoort, K., Diap, G., Festo, C., Johanes, B., Kalolella, A., Mallam, O., Mberu, B., Ndiaye, S., Nguah, S. B., … Goodman, C. (2017). Improving Access To Malaria Medicine Through Private-Sector Subsidies In Seven African Countries. Https://Doi.Org/10.1377/Hlthaff.2014.0104, 33(9), 1576–1585. https://doi.org/10.1377/HLTHAFF.2014.0104
Visser, T., Bruxvoort, K., Maloney, K., Leslie, T., Barat, L. M., Allan, R., Ansah, E. K., Anyanti, J., Boulton, I., Clarke, S. E., Cohen, J. L., Cohen, J. M., Cutherell, A., Dolkart, C., Eves, K., Fink, G., Goodman, C., Hutchinson, E., Lal, S., … Chandler, C. I. R. (2017). Introducing malaria rapid diagnostic tests in private medicine retail outlets: A systematic literature review. In PLoS ONE (Vol. 12, Issue 3). Public Library of Science. https://doi.org/10.1371/journal.pone.0173093
WHO. (2017). A study on the public health and socioeconomic impact of substandard and falsified medical products. WHO. http://www.who.int/medicines/regulation/ssffc/publications/se-study-sf/en/
WHO. (2019). Meeting report of the WHO technical consultation on malaria case management in the private sector in high-burden countries.
WHO. (2020). World malaria report 2020. https://www.who.int/publications/i/item/9789240015791
Yadav, P., Cohen, J. L., Alphs, S., Arkedis, J., Larson, P. S., Massaga, J., & Sabot, O. (2012). Trends in availability and prices of subsidized ACT over the first year of the AMFm: evidence from remote regions of Tanzania. Malaria Journal 2012 11:1, 11(1), 1–11. https://doi.org/10.1186/1475-2875-11-299
Ye, Y., Arnold, F., Noor, A., Wamukoya, M., Amuasi, J., Blay, S., Mberu, B., Ren, R., Kyobutungi, C., Wekesah, F., Gatakaa, H., Toda, M., Njogu, J., Evance, I., O’Connell, K., Shewchuk, T., Thougher, S., Mann, A., Willey, B., … Hanson, K. (2015). The Affordable Medicines Facility-malaria (AMFm): are remote areas benefiting from the intervention? Malaria Journal 2015 14:1, 14(1), 1–11. https://doi.org/10.1186/S12936-015-0904-Z
[1] Median estimate as per WHO methodology to estimate source of treatment for fever among those who were treated using Household surveys. Other estimates for treatment in the private sector are higher (e.g., Rosen et al 2020)
Rights & Permissions
You may use and disseminate CGD’s publications under these conditions.






