Recommended

Blog Post
Last year, CGD set up a working group on antimicrobial procurement that brought together key people from government, academia, civil society, international organisations, and industry, with the aim of finding better policy options that address the limitations of current procurement systems for antimicrobials in low- and middle-income countries (LMIC). These options are aimed at improving access, addressing stewardship, and promoting the development of LMIC-specific products.
This blog outlines two draft recommendations that the working group has been considering: what a global agreement or ‘Grand Bargain’ for antimicrobial procurement should include, and what a global procurement hub could look like.
Overview of the Grand Bargain
Why we need a Grand Bargain
The current system to procure antimicrobials in LMICs fails to provide adequate incentives to develop and sell new products, incentivises overuse by linking profit to volume of sales, and fails to ensure everyone gets access to new antibiotics.
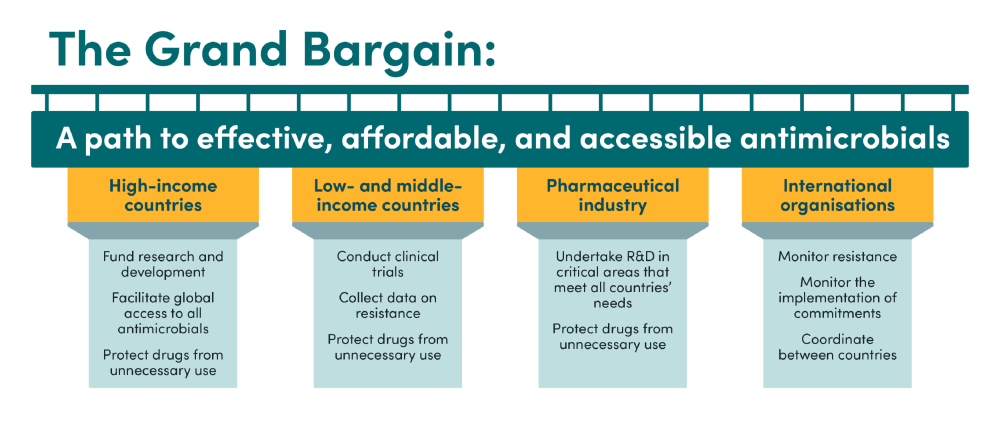
Addressing these issues requires collective action at the highest political level. A "Grand Bargain" on antimicrobial procurement, establishing principles and commitments for all key stakeholders in low-, middle-, and high-income countries in the global antimicrobial market, could be a step in the right direction. The Bargain text could form the basis for a political declaration at the United Nations General Assembly (UNGA) high-level meeting in 2024. This Bargain outlines how countries would get access to all novel antibiotics at affordable prices while protecting the effectiveness of critical drugs and contributing to global antimicrobial research and development at a level commensurate to countries’ means.
Key elements included in the Grand Bargain
National obligations
Wealthier countries should fund new treatments and ensure access to new drugs. In return, all countries should commit to protecting drugs from unnecessary use. Countries should commit to funding and implementing national action plans to protect antimicrobials from unnecessary use and to ensure access. These plans should distinguish between different types of drugs, according to the WHO’s AWaRe classification: ‘Access’ antibiotics should be widely available, 'Watch' antibiotics are for specific infections due to their higher resistance potential, and 'Reserve' antibiotics should only be used as a last-resort option. Countries should enforce responsible use regulations and create national targets for "Reserve" and, where appropriate, "Watch" antimicrobial use, sharing data with the Global Antimicrobial Resistance and Use Surveillance System (GLASS). Countries should ensure purchasing systems generate funds for innovation and fairly divide costs based on ability to pay. Essential new antimicrobials should be purchased without encouraging unnecessary use, potentially through delinking profits from sales volume. Countries should expedite regulatory reviews, establish transparency platforms that disclose incentives for antimicrobial use, and consider demonstration projects in LMICs to determine the best payment systems.
Countries should also commit to establishing a global procurement hub that would ensure access to essential antimicrobials (see global procurement hub below).
Role of multilateral institutions
The WHO should develop frameworks for responsible use regulations and national targets for antimicrobial use, while international financing institutions should provide concessional lending for stewardship and surveillance systems. Bi-annual independent reviews should track progress.
Role of the pharmaceutical industry
The market for antimicrobials should be altered to ensure sufficient incentives exist for antimicrobial R&D and environmentally sustainable manufacturing, and to make it far easier for companies to distribute antimicrobials worldwide. In return, pharmaceutical companies should commit to researching products that meet global R&D priorities. They should make medically important antimicrobials accessible and affordable worldwide, address the needs of all populations, and ensure affordable supply when receiving public or non-profit funding. Companies should work with national governments to create workable regulations to remove incentives for unnecessary antimicrobial prescriptions, including banning sales bonuses and financial enticements for clinicians and medical care workers.
A global procurement hub
An existing entity or entities would improve coordination of antimicrobial procurement and would address market failures limiting access to antimicrobials in LMICs. An initiative such as SECURE or an existing organisation like the Global Fund or Global Drugs Facility would ideally host the procurement hub. The hub would be responsible for product registration, procurement, distribution, market shaping, ensuring unnecessary use prevention, tracking consumption data, providing technical assistance, and offering financial assistance.
The hub would achieve two very important goals: aggregating demand for antibiotics with supply chain problems, and reducing market failures while making the whole market more robust.
Demand aggregation
As a backstop where market systems fail, the hub would aggregate demand and purchase a portfolio of essential drugs and diagnostics from various manufacturers, including innovators and generics firms. This procurement system would be funded with a small mark-up on procurement costs and contributions from donors and would be available to all LMICs and potentially smaller high-income country markets. Potential risks that would have to be mitigated include difficulty in identifying the portfolio of products, demand fluctuations, lack of infrastructure to comply with stewardship standards, and underutilisation.
Making the wider system more robust
As well as pooling procurement, the system would look to shape the antibiotic market by fostering a robust manufacturing system, ensuring affordable pricing, promoting eco-friendly manufacturing practices, guaranteeing quality assurance, and supporting innovation for drugs meeting the needs of LMICs. These goals would be incorporated in any procurement approach, prioritising market sustainability over the lowest bidder.
Building consensus
The working group has been built on existing proposals and initiatives which people from across the AMR space have kindly taken the time to share with us during the last 18 months. Indeed, more than 60 percent of people that we interviewed as part of our Landscape Review highlighted the need for a better system for international co-ordination. We are working to incorporate feedback from a large number of experts in government, industry, academia, civil society and elsewhere, with the aim of creating final proposals that all key stakeholders can support. If you have ideas of what should be included or excluded from these two recommendations, please email Morgan Pincombe.
This work will all feed into our final report, which will be released in late September this year, to coincide with the high-level meetings at UNGA 2023. This will outline what a high-level political agreement on antimicrobial procurement could look like. We will also outline how key features of the bargain can be implemented, especially operational changes that we believe need to be made at the global, regional, and national levels, such as the global procurement hub. Antimicrobial resistance is already a major global killer. Without concerted action to improve access, innovation, and stewardship of these crucial drugs, the problem will only get worse. As the topic returns to the UNGA high-level meeting agenda for only the second time in 2024, the world should come to together to take the concerted steps needed so that we can keep these essential treatments working.
Disclaimer
CGD blog posts reflect the views of the authors, drawing on prior research and experience in their areas of expertise. CGD is a nonpartisan, independent organization and does not take institutional positions.
Image credit for social media/web: Adobe Stock