Recommended
Blog Post

Event
This month, Ghana and Nigeria became the first two countries to approve the use of R21, a vaccine that may have game-changing effectiveness in protecting people from malaria. The emergence of effective vaccines promises a new golden age of success in the fight against malaria, just as such efforts had appeared to slow and even reverse. However, such promise must be neither squandered nor overplayed. A deficiency of timely, policy-relevant evidence hampered decision-making about the world’s first malaria vaccine—RTS,S—and cheap and highly-effective technologies, such as insecticide-treated bed nets, have still not reached full coverage. Health officials in malaria endemic countries and donor organisations, such as the Global Fund to Fight AIDS, Tuberculosis and Malaria (the Global Fund) and Gavi, the Vaccines Alliance, (Gavi), must make difficult decisions regarding how best to invest limited resources in this new age of malaria control and, hopefully, elimination.
In this blog, we call for integrated, national priority setting mechanisms to be in the driving seat in malaria decision-making; supported by scenario-based, context-sensitive guidance from the World Health Organization (WHO) or regional bodies, locally customisable health technology assessment (HTA) models, and well-coordinated donors; and provided with pragmatic evidence that is focused on meeting these decision maker’s needs. Through this, we can ensure the world realises the full potential of malaria vaccination.
Tame the inner magpie, with evidence
Like the magpie, we all like shiny, new things. RTS,S understandably caused much excitement, despite being only moderately effective, with between 30-40 percent reduction in malaria cases (depending on specific circumstances). Early evidence suggests that R21 is significantly more effective, perhaps preventing as much as 80 percent of malaria cases in those vaccinated—the first malaria vaccine to break WHO’s target of 75 percent efficacy. While this first trial is relatively small (n=450), findings from a much larger trial (n=4800) are due soon.
There are, however, a range of existing cost-effective tools in the box for malaria control, and alternative healthcare investments that have not been fully rolled out. It is vital that vaccines build on these strong foundations and do not undermine them. In malaria, this means vector control (including using appropriate bed nets), early diagnosis, treatment using an artemisinin combination therapy (ACT), and intermitted preventative treatment. Resources spent on malaria vaccines are likely to take away from resources spent on these foundational interventions, and this opportunity cost must be carefully considered. For decision-makers to make the right calls on whether or how much to spend on RTS,S now, expand conventional measures like bed nets, or hold out for R21 roll-out, the right evidence is needed at the right time, supported by rigorous national priority setting systems, with regional and global support (see Figure 1).
Figure 1. Achieving an effective, evidence-informed R21 vaccine rollout
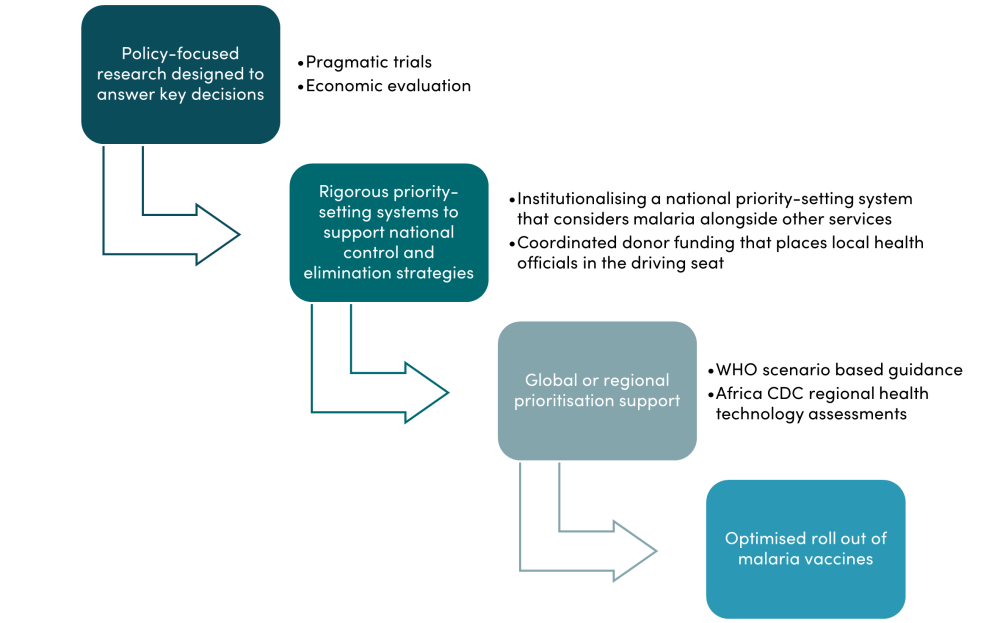
Policy-focused research
Identifying clear policy questions early is essential to maximising the effectiveness and timeliness of research, as illustrated by this decision framework for malaria vaccines. The R21 phase three randomised control trial (RCT) will provide vital efficacy and safety data, but will not be sufficient to answer many real world challenges and choices that policymakers face. For instance, the data will not clearly evaluate whether R21 should be prioritised over other effective malaria interventions, such as bed nets.
Clear identification and articulation of the relevant policy questions through policymaker and donor workshops is needed. These can be used to supplement the RCT with additional pragmatic trials, including built-in economic evaluations and economic and epidemiological modelling studies that can extrapolate beyond trial results. This means collecting cost data and powering the study, not just with efficacy in mind, but also comparative effectiveness against relevant competitor technologies in real world settings. For future vaccines, funders and researchers should do this from the beginning, with trials informed by early HTAs, target product profiles, policymaker and donor engagement workshops to establish what key questions need to be answered from the outset.
Research can also help countries to develop targeting plans to prioritise those who receive R21. It is not uncommon for implementers to aim to provide malaria interventions to all at-risk populations—and since malaria transmission is typically higher in more remote populations, this can result in those at greatest risk being last in line. The importance of targeting malaria interventions has been well established by multiple trials and modelling studies, and so to realise the full potential of R21, it is essential that it is targeted to those most at risk. Routine case or serological survey data combined with information on environmental risk factors can effectively identify hot spots—and this can be supplemented by geographically differentiated cost-effectiveness analysis to help realise the full potential of malaria vaccines, by getting them to the right people.
Integrated priority setting systems at both national and global levels
To decide whether and to what extent to fund a roll-out of the R21 vaccine, countries need rigorous evidence-informed prioritisation processes. However, fragmentation of the health system can undermine a country’s ability to appropriately value malaria vaccines in two ways. Firstly, malaria control is often led by a National Malaria Control Programme (NMCP), separate from other health services, and, in some cases, heavily funded by donors, such as the Global Fund. This makes consideration of trade-offs with other health services rare and challenging. Secondly, vaccine policymaking often has a dedicated process, typically linked to a National Immunisation Technical Advisory Group (NITAG) and backed by Gavi. In the short term, NMCPs and NITAGs should work together to review the value for money of malaria vaccines. In the long term, countries need to be supported to develop a single national priority setting system, that can consider the value for money of malaria vaccines versus all other health system spend.
This challenge is mirrored in the multilateral architecture. Until the approval of RTS,S, the Global Fund and Gavi had clearly defined separate roles in the global health architecture. RTS,S hasn’t just blurred the lines, it’s obliterated them, with Gavi announcing in December 2021 that it would move into malaria and provide $156m for RTS,S. This decision matters as Gavi will consider the vaccine in comparison to other vaccines in its portfolio, while the Global Fund would make comparisons with other types of interventions to combat malaria. To demonstrate the confusion this may cause, it is quite possible that future malaria vaccines such as R21 could be considered cost-effective within the Global Fund’s portfolio but not within Gavi’s, for example.
The idiosyncrasy of malaria vaccines being potentially cost-effective for one multilateral fund but not another, reveals that a new comprehensive framework is needed for how global health funders and countries work together on a single coherent approach to evidence-informed priority setting. As part of this framework, it is important that international financing does not displace domestic financing or undermine efforts to build strong health systems. We outline such an approach in a recent paper, arguing that domestic funding should focus on core services, and donor financing should ‘top up’ services at the margin.
Regional and global prioritisation support
Global guidance from WHO is valuable but it often lacks the nuance required to fully support national malaria control programme managers, who need to prioritise between interventions based on local budgets, cost-effectiveness, epidemiology (including transmission patterns and demography), entomology, population preferences and existing service coverage. A one-size-fits-all conclusion about intervention cost-effectiveness is not helpful. Instead, guidance could do more to support local decision-making by laying out likely malaria intervention prioritisation under various scenarios and budgets. Recent WHO guidance on how to prioritise different kinds of bed net in the face of limited resources is a welcome improvement. WHO guidance on R21 would be most useful to policymakers if it builds on this scenario-based approach.
Regional bodies might also be able provide technical guidance, with a more nuanced approach tailored to local contexts. The African Centres for Disease Control and Prevention, soon to be under new leadership, is well placed to support African countries with guidance on malaria vaccination, including consideration of health economics evidence.
Recommendations
R21 represents an exciting milestone in the history of malaria control. To realise the full potential of the vaccine, we must focus evidence generation on the informational needs of key decisions, support integrated national priority setting, and optimise targeted use. In pursuit of this aim, we recommend that:
- WHO and regional health institutions should avoid a one-size-fits-all global recommendation on R21 and focus on developing structured guidance on the cost-effectiveness of R21 and other malaria interventions in a typology of settings and resource levels.
- Funders should back a range of research that fully address relevant policy questions for R21 and for future vaccines. This includes pragmatic trials and ensuring economic evaluations are designed into trials of new technologies from an early stage. If financing vaccine provision, funders should take care to support and not undermine an integrated national approach to setting health priorities.
- Low- and middle-income decision makers should, where possible, use evidence-informed prioritisation processes to avoid the hype, get a clear view on vaccine value for money, and target high priority groups. They should also seek donor support for these processes and consider if a marginal aid approach is suitable.
- Researchers should ensure studies address clear policy questions and results can be extrapolated beyond the trial setting. Economic evaluations should be integrated into trials at the design stage.
The creation of a highly effective vaccine to combat one of the world’s oldest and most deadly diseases is cause for much excitement. Yet we must approach this opportunity with a cool head and a determination to make the most of R21 as part of a holistic approach to malaria control and elimination, within effective health systems.
Disclaimer
CGD blog posts reflect the views of the authors, drawing on prior research and experience in their areas of expertise. CGD is a nonpartisan, independent organization and does not take institutional positions.





