Recommended

POLICY PAPERS
In many low- and lower-middle-income countries (LMICs) where disease burdens are highest, health supply chains function poorly, resulting in frequent stockouts and a high prevalence of substandard and even falsified medications, both of which undermine the effectiveness of treatment regimens and raise the risk of anti-microbial resistance. In response to these concerns, the global health initiatives have stepped up their efforts to improve supply chain management.
At the same time, a growing number of rich country pharmaceutical companies are investing in digital technologies in response to new regulations—including the US Drug Supply Chain Security Act (DSCA) and the EU’s Falsified Medicines Directive—that require producers and distributors to “track and trace” the movement of medicines through the supply chain at the package-level.
In a new paper, my coauthor Denise McCurdy and I explore whether traceability offers a realistic solution to some of the problems found in LMIC health supply chains. Drawing from interviews with over 30 experts, we discuss the promise that traceability holds for LMICs, while also highlighting hurdles to successful implementation, including the need to invest in a reliable data-sharing infrastructure, coordinate around the use of a single product ID standard, and get buy-in from a critical mass of participants. Because many of these challenges are political rather than technical in nature, governments with a strong political commitment should be able to overcome them.
The state of LMIC health supply chains
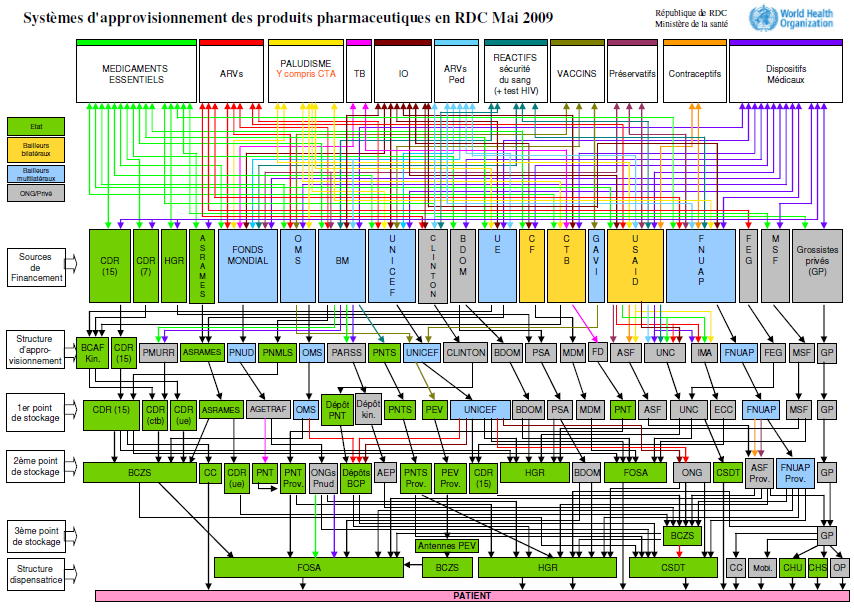
Unlike in rich countries, where health supply chains are managed almost entirely by the private sector, in LMICs “public, private, and non-governmental organizations (NGOs) co-exist as channels of distribution for medicines, with various interconnected flows between the three channels.” The result is often a convoluted tangle of relationships that national authorities struggle to manage. Figure 1, which maps the medical supply system in the Democratic Republic of Congo, illustrates just how complex these arrangements can be.
Figure 1. Pharmaceutical supply systems in the Democratic Republic of Congo, May 2009
The complexity and opacity of LMIC health supply systems creates inefficiencies and makes them vulnerable to corruption and product diversion: without effective monitoring, each handoff on a supply chain represents an opportunity for diversion, and the path that imported medicines follow from customs agent to local health center often includes many intermediaries. For example, in Kenya medicines can change hands five to seven times before reaching local clinics.
Product diversion contributes to the high frequency of stock-outs in LMICs, along with poor demand forecasting. It also raises the risk that substandard medicines will enter the health system, as wrongdoers try to cover their trail by substituting in substandard or (more rarely) falsified medicines in their place.[1]
Traceability to the rescue?
As the name suggests, traceability or “track and trace” initiatives allow actors on a supply chain to determine where a product is at any given time (tracking) and where it came from (tracing). The approach starts with the process of serialization, in which a manufacturer assigns a unique identifier to each product that it ships using a two-dimensional (2-D) barcode that other supply chain actors can scan to obtain information about the product and record when it changes hands. The result is a digital trail of information tied to each package that records its origin, path through the supply chain, and other attributes.
The primary benefits offered by traceability for health supply chains include:
-
Verifying the origin of goods: The ability to trace a product back to its origin can help dispensing agents (and potentially even patients) verify that a medicine is what its label claims. The ability to track the movement of individual products through a chain of custody can make it easier to detect when a medicine is diverted away from its intended destination.
-
Improving product safety and facilitating recalls: Having the ability to quickly identify the source manufacturer of a contaminated medicine could make it easier to halt production and process recalls, reducing both costs and the risk of illness.
-
Improving supply and demand forecasts for procurement: Traceability data could provide greater visibility over supply and demand patterns for a given product and improve demand forecasting. Procurement agencies could use this information to forecast their commodity needs and anticipate stockouts.
While these potential benefits are significant, traceability systems cannot address concerns about drug quality on their own. For instance, while traceability can make it easier to trace substandard products back to their source, quality assurance testing is needed to determine what medicines are substandard in the first place. Post-market surveillance is also needed to ensure that consumers are receiving high-quality products. Finally, an effective traceability system requires a clear legal framework supported by a culture of compliance and enforcement. For these reasons, traceability should be treated as just one element of a broader, holistic effort to enhance supply chain integrity.
Making the (business) case for traceability
Developing a traceability system is a huge logistical endeavor that requires significant upfront investment: manufacturers must install new machinery and software, while downstream actors must buy scanning equipment and train employees how to access and use serialization data. In addition, an IT infrastructure must be developed to enable data-sharing between different supply chain actors.
Given the substantial investment required, governments will need to be convinced of the approach’s benefit before making the leap. The global health community has made impressive strides in recent years raising awareness of these benefits and working with LMIC governments to lay the groundwork. But there is still a lack of information about which traceability approaches work best and in what context.
The donor community can help fill this gap by funding pilot projects that use different traceability models to measure their impact. For example, USAID/PEPFAR and the Global Fund could design a series of pilot projects to assess the impact of introducing a specific traceability system on leakage, savings, and the share of products delivered on-time-in-full (OTIF), as well as the operational and capacity requirements for implementation. For each pilot, an evaluation team would measure a baseline of metrics ahead of implementation and assess progress on those metrics on a monthly basis.
The findings of such an evaluation would help USAID and the Global Fund better assess the costs and impact of introducing traceability and what prerequisites need to be in place. Once donors have gathered enough evidence to estimate a return on investment measured in lives saved and costs reduced, they can then work with committed LMIC governments to translate that knowledge into action.
[1] Although substandard and falsified medicines are often lumped together, they represent different problems. Substandard medicines are made by registered manufacturers but do not meet quality standards, usually because they contain the wrong amount of active ingredient, have been degraded in transport, or have expired. Falsified, or counterfeit, medicines are sold in packages that are deliberately designed to deceive consumers. They may contain an amount of active ingredients that is either dangerously high or ineffectively low, or contaminants, or no active ingredient at all.
Disclaimer
CGD blog posts reflect the views of the authors, drawing on prior research and experience in their areas of expertise. CGD is a nonpartisan, independent organization and does not take institutional positions.
Image credit for social media/web: Gatis Gribusts/Flickr