Recommended
Executive Summary
The global effort to control the COVID-19 pandemic has seen an exceptional allocation of public and philanthropic funds to advance the development of diagnostics, therapeutics, and vaccines as quickly as possible. While critical, even these significant commitments represent only a “down payment” on a price tag that could eventually exceed $50 billion just to scale the production of vaccines to control this global pandemic—amounts that cannot be raised through traditional donor and philanthropic commitments.
High-income countries (HICs) can afford to compete for products, and if their taxpayers are willing to contribute, traditional donor funding approaches can help low-income countries (LICs) through mechanisms such as the GAVI-proposed Advance Market Commitment (AMC). However, billions of poor people who live in middle-income countries (MICs) ineligible for donor funding are at risk of being left out. Any exclusion will undermine the effort to control the virus. Further, MICs are key actors in the global supply and production chain, and we will require an unprecedented level of collaboration between governments and with industry to develop and rapidly manufacture global supplies of a vaccine. No country has all the science, equipment, and capacity on its own soil to research, develop, manufacture, and supply a vaccine to all its citizens, let alone the whole world.
The world is certain to experience other pandemics—it is a matter of when, not if. The important question now is whether the battle against COVID-19 will strengthen or exhaust our ability to respond again in the future. The world faces a unique challenge, but it also has a unique opportunity to craft solutions that not only address the challenge of providing effective vaccines against COVID-19 but also are replicable and sustainable. To do so will require us to abandon the binary distinction of health goods as “market driven” or “charitable.” Instead, we must create incentives and pathways for governments to work together and with the private sector to tap its enormous reserve of capital, talent, and technology.
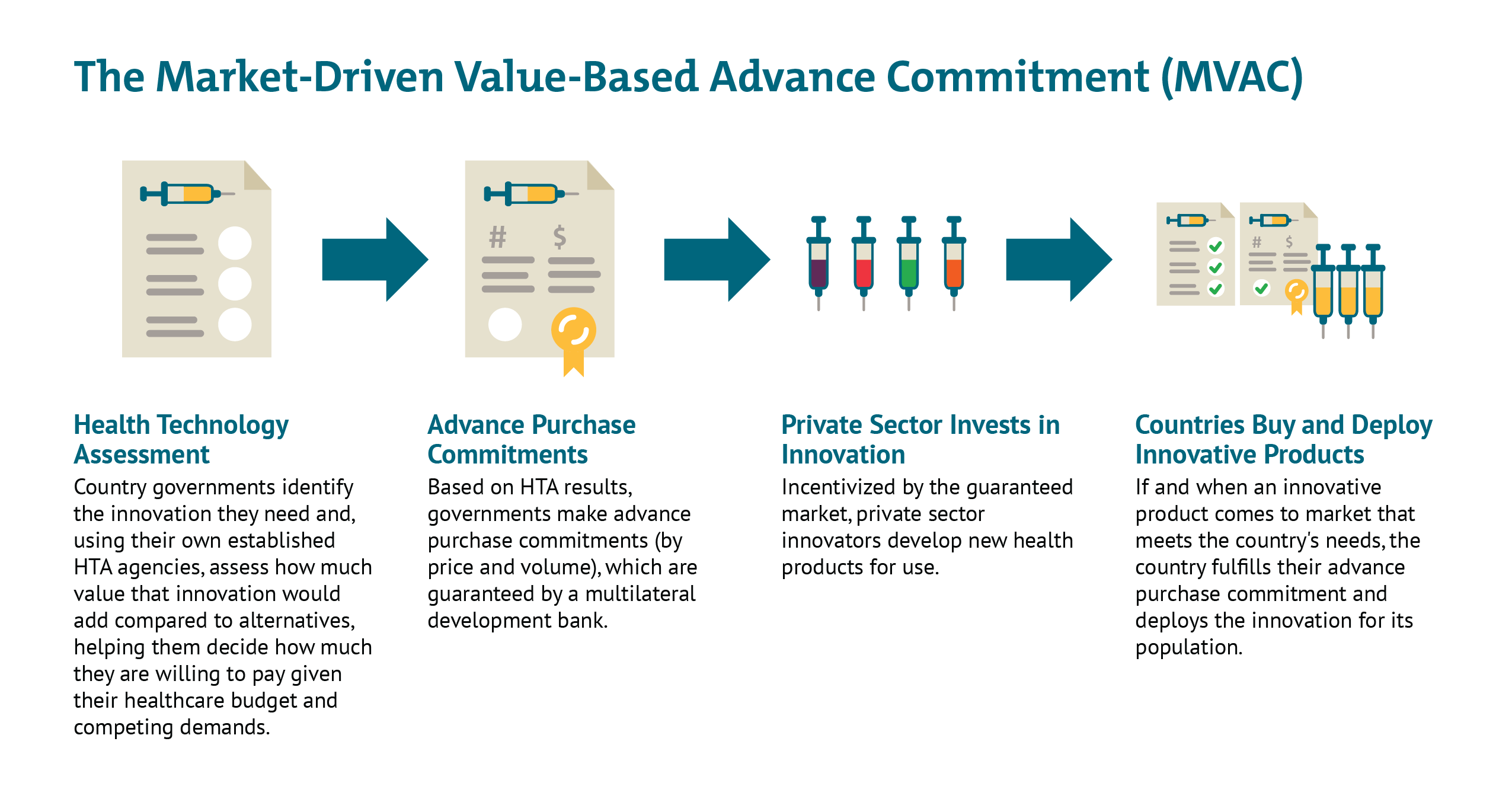
In this note we propose a new mechanism that would address these challenges. The Market-Driven Value-Based Advance Commitment (MVAC) is designed to pull in money from the private sector to help fund the R&D and manufacturing scale up by explicitly monetizing future expected demand from HICs and MICs. The MVAC differentiates the price according to efficacy, thereby incentivizing development and use of vaccines with higher rates of disease prevention, while ensuring that vaccines are available at manufacturing cost for LICs. By pooling commitments and pre-agreeing vaccination priorities, countries avoid destructive beggar-thy-neighbour policies to pre-empt supplies, instead sending clear signals to market entrants.
Without consideration of value, HICs and MICs risk getting locked into purchasing inferior or cost-ineffective products that divert scarce resources from other potential COVID-19 responses, including potential therapies, non-pharmaceutical interventions like testing and contact tracing, and superior vaccines. If all the funding flows to the first entrant, the second or third entrants may be boxed out of the market—even if their product is of better value or more efficacious.
Given the importance of having enough manufacturing capacity to supply successful vaccines, a complementary advance purchase mechanism that operates alongside the MVAC could be set up with donors and governments entering into contracts with potential manufacturers who would receive licenses from the innovator, with an initial contract component entering into force immediately to build and hold manufacturing capacity before a vaccine has come to market.
By rewarding performance, the MVAC can increase the likelihood that all the world’s citizens gain access to effective vaccines that all countries, regardless of income level, have a stake in.
Context
Over 100 vaccines are under development against COVID-19 today but unless we take international action to ensure that most of the world’s population have access, we cannot realize the global goal of controlling the pandemic. Where a circulating virus anywhere poses the threat of reintroduction everywhere, timely introduction and broad coverage are not just issues of equity but are critical to an effective response.
The first challenge to global and timely access is the paucity of public funding for financing an issue of this magnitude. In support of the recently launched Access to COVID-19 Tools (ACT) Accelerator, philanthropies and governments have committed nearly $8 billion to support the development of diagnostics, therapies, and vaccines, but they recognize that this is only a “down payment.” Even when we have an effective vaccine, tens of billions of dollars will be required to scale up the manufacturing of doses needed. Biopharmaceutical companies are nervous about the reliability of future markets and respect of IP rights, and are reluctant to front the capital investments needed,[1] and there is no credible public-only alternative through which to coordinate vaccine development, manufacture, and distribution.
A second challenge is the pressure governments face to prioritize their own citizens over a globally coordinated allocation plan, even if that global allocation might better serve the global goal of controlling the virus. The capabilities and resources to develop vaccines are concentrated in just a few HICs and MICs, where governments, under immense public pressure, may adopt both financial and legislative measures to restrict global access to vaccines, or vaccine components, manufactured in their jurisdictions. Without legal constraints, pre-approval manufacturing agreements or domestic export policies can still restrict international availability.[2] To overcome the instinct and public pressure to prioritize national self-interest, governments need to assure their public (and themselves) that a coordinated plan will better meet their priorities, be that volumes, price, or something else.
The third challenge is that many MICs and LICs are at risk of being deprioritized for supplies even as global leaders are committed to leaving no one behind. Most countries are not funding their own vaccine candidates, have no or only limited manufacturing capacity, do not offer an attractive financial market, and are underrepresented at the global tables. A sustainable solution that recognizes and responds to the different circumstances and needs of HICs, MICs, and LICs is needed.
A Public-Private Disconnect?
At the heart of this problem is the fact that there is no coherent path to align public and private investment along the vaccine development value chain from laboratory to global market. Public and private efforts to fund and collaborate are more aligned in upstream R&D, where the main risks are scientific. As the products advance, many complementary efforts are required to accelerate the development and manufacture of accessible and desirable vaccines, and to realize the optimal distribution needed to control the pandemic as quickly as possible. However, without credible market pulls that includes explicit expectations with regard to global access and for whom, companies that might have the capital to resolve the funding bottlenecks are likely to wait for more push funding, unable to see a pathway to a reasonable return between the high-costs of development and manufacturing to global scale and the volatile and politically charged market.
To sustainably resolve this, we need to create mechanism(s) to improve the predictability of the companies’ market returns so as to mobilize their investment in development and manufacturing capacity. And, in tandem, we need a mechanism(s) to mobilize additional suppliers to prepare to scale manufacturing to global demand in parallel with the innovators.
Does a Solution Exist?
In 2019, we proposed a Market-Driven, Value-Based Advance Commitment (MVAC) to incentivize private sector investment for a universal drug regimen for tuberculosis (TB), a condition that is mostly prevalent in MICs and LICs. A modified version of this MVAC, that includes HICs, offers a potential global solution for the current crisis, incentivizing COVID-19 vaccine innovators to make investments by improving the credibility and predictability of all the markets. With more certainty about returns in HICs and MICs, companies are more likely to agree to offer the LICs the lowest tier subsidised prices either directly or via voluntary licenses to local manufacturers through conduits such as the Medicine Patent Pool. A parallel and linked advance purchase agreement could help mobilize those license recipient suppliers to invest early in expanded manufacturing capacity.[3]
Incentives for accelerated development and commercialization
The MVAC is based on the AMC—a concept piloted with pneumococcal vaccines in 2009—with some important adaptations. The advance commitments in the MVAC case are built on value assessments done by national payers that are indexed to their ability to pay for a new product. Countries make a commitment to purchase—without having to put up the money now—with their contributions scaling as a function of the value of the vaccine to their health system and ability to pay. The poorest countries would pay the least—possibly zero—and their contributions could be met with full or partial donor support through an organization like GAVI.
Assuming more than one vaccine proves sufficiently efficacious, the vaccine that best meets the preferred product characteristics (as detailed in the WHO Target Product Profile or TPP) would receive a greater share of the total revenue commitment. However, the mechanism would allow inclusion of more than one product meeting minimal TPP requirements. As was the case with the pneumo AMC, provision will be made for a product offering more suitable characteristics for implementation in different contexts and populations. It also takes account of the likelihood that the first vaccine to get a product license may not meet all needed criteria (in terms of overall efficacy, or method of administration) and multiple vaccines may ultimately be necessary to address challenges of efficacy and tolerability in sub populations or to address field conditions.
The value assessment element of MVAC allows for the same product or indeed different products to command different prices and hence market share across different country contexts as they may meet local needs at differing levels (perhaps because of a differential safety profile; their effect on younger vs elderly multi-morbid populations; the need for cold chain; or administration complexity). In addition to accommodating population heterogeneity through subgroup analysis, such an integrated health economic and dynamic disease transmission model[4] would also allow for comparisons to be made against future counterfactuals of different levels of herd immunity or more effective treatment regimens coming about, when assessing the future value of a vaccine.
An MVAC for a COVID-19 vaccine would have the following attributes:
- Early health technology assessment (HTA), (building on countries’ existing national and regional HTA processes, such as UK’s NICE, Australia’s PBAC, or Thailand’s HITAP), would be used to understand how helpful a vaccine would be in different country contexts and determine the ability of each country to pay. The results would then be adjusted downwards for relevant push funding and “locked in” to provide overall market predictability.
- MVAC could use a financial intermediary like a multilateral development bank for MICs or HICs’ national bank reserves or holdings of government bonds, to underwrite countries’ own value-based advance market commitments, so countries do not need to put scarce resources aside until an effective product comes to market.
- All countries could participate in the mechanism to guarantee a large total market commitment, but their contributions to the total market would vary based on their respective ability to pay and population sizes.
- Governments would construct the MVAC to offer multiple value-based entry market commitments (country-specific tiered prices for guaranteed volumes) to multiple developers that meet the minimum effectiveness threshold (as per the WHO TPP) as an incentive to keep many different potential innovators in the game post launch. This would hedge risk against late failure of one or more early candidates and against the possibility of safety risks after widespread deployment requiring restricted use or withdrawal from the market of the first entrant.
- As a condition of accessing this guaranteed market, governments would require the successful innovator(s) to license their vaccines out to other suppliers at low or zero cost, helping facilitate widespread scale-up across all countries, but in particular in LICs.[5]
Incentives for scaling manufacturing
In anticipation of a widely licensed vaccine, an advance purchase mechanism that complements the MVAC could be set up with donors and governments entering a two-part contract with potential manufacturers with licenses from the innovator.
The first part of the contract would enter into force immediately to build and hold manufacturing capacity for production of a vaccine (at a flat rate possibly determined through an auction mechanism), before a vaccine has come to market. Public monies can fund this, and such public investment will be reflected in a discounted price. The mechanism could solicit potential suppliers through open bidding to ensure they receive manufacturing capacity at competitive prices. It will require careful hedging of investment to align to different vaccine platforms that might be most easily adaptable if the front runners fail. While this might be more straightforward for conventional vaccine platforms, it may require significant additional upfront investment to build up large-scale production of innovative nucleic acid vaccines. High-quality manufacturing, meeting standards agreed by regulators, geographically distributed, and the innovator company—and vigorously enforced by regulators—would be a key requisite.
The second part of the contract would enter into force once a vaccine is approved and licensed and the reserved manufacturing capacity is put into use on a competitive pricing basis. This is a cost which could be internalised by the participating innovators (as per conventional contract manufacturing organization [CMO] contracts) and reflected, this time as a positive, in the final value-based price.
How to distribute manufacturing plants and manufacturing supplies globally, including across sub-Saharan Africa, to ensure seamless and efficient transport and access would also need to be explored.
MVAC for a COVID-19 Vaccine
The MVAC de-risks the commercial market, encourages voluntary licensing early to crowd in additional suppliers, and offers an avenue for widespread, rapid uptake across countries with divergent abilities to pay. It incorporates flexibilities to reward value—and ensures we continue to incentivize investments in the best (and safest) possible products.
The MVAC signals to industry that the market values their up-front investments and those investments have the potential to pay off down the line in a way that reflects the value of their product.
Perhaps most importantly, the MVAC differentiates the price according to efficacy, drawing on countries’ established HTA capacities, and incentivizing development and use of vaccines with higher rates of disease prevention, while ensuring that vaccines are available at manufacturing cost for LICs.
Without consideration of value, countries risk getting locked into competing to purchase inferior or cost-ineffective products that divert scarce resources from other potential COVID-19 responses, including potential therapies, non-pharmaceutical interventions like testing/contact tracing, and later, superior vaccines that may be boxed out of the market if all funding immediately flows to a first, poor-value or minimally efficacious entrant.
Finally, beyond the immediate challenge at hand, a MVAC for COVID-19 vaccine could set a sustainable precedent for how to combat future emerging challenges, demonstrating to developers how a market could exist for high-value innovations and encouraging them to invest aggressively to tackle global challenges, crowding in private capital that governments may find hard to raise alone. Recent fundraising cycles to tackle antimicrobial resistance and now COVID-19 have shown this to be a challenge.
Next steps
A flurry of activity is already underway to address the vaccine financing, manufacturing scale up, and allocation issues for COVID-19, but none are taking a value/health benefit-based approach. With an AMC being discussed for LICs, and nationalistic and protectionist tendencies across HICs and some MICs, it is urgent we send coordinated and clear incentive signals to the public and private actors who we need invested in the research, development, manufacture, and delivery of COVID-19 vaccines.
We call on governments, philanthropic organisations, and key international bodies to work together with the private and nonprofit sectors to design and implement a benefit-based AMC based on our MVAC mechanism.
These unprecedented times require novel solutions and bold, unprecedented action.
[1] “If the industry does not know if there will be a market in 18 months, [it] cannot carry all [the costs]. Industry alone can’t provide all the investment needed now for billions of doses,” David Loew, EVP, Sanofi-Pasteur, FT April 20, 2020.
[2] “The danger is that richer nations will buy up the supply for their own use or prevent exports of vaccines developed within their borders as countries scramble to protect their citizens or stockpile for future outbreaks. Another concern is that manufacturers might restrict sales to the highest bidder.” Seth Berkeley, NYT Op-ed, April 29, 2020.
[3] We propose explicit accommodations for the LIC market be incorporated into the MVAC but a complementary and separate GAVI AMC developed to service LICs could be an alternative approach to consider.
[4] For examples of health economic evaluation using dynamic disease models see here for dengue vaccine introduction and here and here for a more general exposition of the issues.
[5] The LIC commitments could be paid for by global donors through Gavi/IFFIm and maybe cost plus AMC. Donors might offer further support for delivery within countries with weak health systems
Rights & Permissions
You may use and disseminate CGD’s publications under these conditions.