Recommended
Key messages
- Deploying viral vector vaccines (similar to AstraZeneca (AZ) and Johnson & Johnson (J&J)) in Ethiopia in 2021 would have been a highly cost-effective intervention, averting between 180,000 and 440,000 disability adjusted life years (DALYs) over five years. Using an mRNA vaccine (like Pfizer) would have provided health benefits approximately 20 percent greater, whilst the health impact from an inactivated virus vaccine (like Sinopharm) would be around 30 percent lower.
- Using a viral vector vaccine may have resulted in cost savings to the Ethiopian health sector due to reducing COVID-19 care and treatment costs. The savings could be as much as $200 million over five years. In contrast, an mRNA vaccine (with prices similar to Pfizer) would have cost the health system between $150 million and $330 million. A Sinopharm-like vaccine would be about 2.5 to 4.5 times more expensive again.
- Our study found that viral vector vaccines would have been highly cost-effective in Ethiopia. A Sinopharm-like vaccine would not offer good value for money in Ethiopia. At between $320 and $1,200 per DALY averted, a Pfizer-like vaccine would only be cost-effective in the most optimistic scenarios we modelled, and only if more cost-effective vaccines are not available.
- Vaccine price was a more substantial driver of cost-effectiveness than any other single factor. Differences in the costs of different ways to deliver vaccines matter far less.
- In terms of delivery strategy, targeting vaccines firstly at older people and increasing the speed of roll-out improve the cost effectiveness of the COVID-19 vaccine programme.
Background
COVID-19 radically changed most people’s lives in 2020, including across Africa. In Ethiopia, the disruption started with school closing, and other restrictions soon followed. Despite these efforts to contain the virus, Ethiopia—like almost all countries—suffered significant health impacts. It has recorded seven thousand deaths by the end of April and likely many more that were not recorded. In 2021, vaccines offered a ray of hope, but early supplies were concentrated in high- and middle-income countries. Most low- and many lower-middle-income countries struggled to obtain doses, both because the vaccine were difficult to source and because they were expensive. The Ethiopian government spends on average about $23 per person per year on healthcare, so spending more than $10 per person to procure and distribute COVID-19 vaccines is a significant investment that requires careful consideration. To inform future decisions, we undertook a Health Technology Assessment to assess whether the vaccines offered good value for money for Ethiopia in 2021. We also looked at the best ways to distribute the vaccine and the effectiveness of targeting specific age groups.
Policy questions
- Which vaccines should Ethiopia purchase?
- How should these vaccines be distributed?
- What age groups should be targeted?
Methodology
We looked at four hypothetical vaccines, designed to be similar to existing vaccine that Ethiopia was considering purchasing. We modelled each from a health system perspective, with coverage rates between 25 percent and 100 percent, or only individuals over the age of 50. We performed the modelling in 2021 based on epidemiological conditions in Ethiopia Q2 of 2021. We examined viral vector vaccines similar to AstraZeneca (AZ) and Johnson & Johnson (J&J), an mRNA vaccine like Pfizer, and an inactivated virus vaccine similar to Sinopharm. Table 1 describes the base price, dosing, and baseline efficacy we used in our cost-effectiveness calculations.
Table 1. Summary of characteristics used to model each hypothetical vaccine
|
Vaccine |
Base price ($) |
Doses needed |
Efficacy (%) * |
|---|---|---|---|
|
Viral vector vaccine 1 (AstraZeneca - like) |
3 |
2 |
75 |
|
Viral vector vaccine 2 (Johnson & Johnson-like - like) |
10 |
1 |
66 |
|
Inactivated virus vaccine (Sinopharm - like) |
30 |
2 |
51 |
|
mRNA vaccine (Pfizer - like) |
17 |
2 |
90 |
*As defined by the reduction in symptomatic infections for the person inoculated
We then looked at three different methods for distributing the vaccines:
- Fixed posts. These are mainly health facilities and other appropriate locations.
- Vaccination campaigns. These are used to visit large population centres in a shorter time and involve going to people to inoculate them.
- Outreach posts. These are set up in remote or hard to reach areas where travel times to a health facility are long or there is limited access to health services.
We estimated the cost of freight (how much it cost to ship the doses from the manufacturing site to Ethiopia), at $0.90 per dose, and the delivery cost at $5.29, $6.63, and $7.13, for fixed posts, campaigns, and outreach posts, respectively.
We then looked at two base case scenarios:
- Slower scenario: 10 percent of Ethiopians will be vaccinated by the end of 2021, 50 percent vaccinated by the end of 2022, and 80 percent vaccinated by the end of 2023.
- Faster scenario: 20 percent of Ethiopians will be vaccinated by the end of 2021 and 80 percent vaccinated by the end of 2022.
While we know that COVID-19 vaccines reduce transmission, it is not clear to what extent. For this reason, we modelled all results in two ways. Our disease model output presumed the vaccines have no benefit in reducing transmission and only help the person vaccinated to avoid symptomatic illness. Our infection model presumed that the efficacy of the vaccines against infection transmission was the same as the efficacy against disease. These should be treated as the upper and lower bounds for the vaccines benefit.
We measure benefits using an incremental cost-effectiveness ratio (ICER): the difference in cost between two interventions, divided by the difference in their effect. ICERs effectively tell you how much you are paying for every unit of health benefit. The comparator scenario in this study was no vaccination. The more expensive vaccines would seem less cost-effective if compared to the cheaper inoculations.
All results are based on modelling of COVID-19 vaccines before the Omicron variant became dominant and looking in part at decisions that could have been made in early 2021. There are important generalisable lessons going forward for both Ethiopia and elsewhere.
Policy question 1: Which vaccines should Ethiopia purchase?
Unsurprisingly, all vaccines were shown to have large and positive health benefits. These benefits varied greatly, however, according to the speed of vaccine delivery, and the vaccines impact on transmission (which the infection model accounts for, but the disease model does not). Table 2 shows the DALYs averted per vaccine. These results are primarily driven by the efficacy of the vaccine, with a Pfizer-like vaccine having the greatest health benefit and a Sinopharm-like vaccine the least. The two viral-vector vaccines being in between.
Table 2. DALYs averted per vaccine
|
Vaccine |
Disease slower scenario |
Infection slower scenario |
Disease faster scenario |
Infection faster scenario |
|---|---|---|---|---|
|
AZ-like |
179,521 |
347,821 |
255,038 |
433,989 |
|
J&J-like |
216,561 |
350,917 |
272,180 |
439,578 |
|
Sinopharm-like |
123,106 |
251,203 |
169,222 |
317,690 |
|
Pfizer-like |
229,367 |
414,209 |
319,263 |
537,226 |
The health system cost also varied substantially based on both the vaccine, the model used, and how quickly the vaccines are rolled out. As vaccines can offer substantial savings by reducing hospital stays and other COVID-19-associated costs, some vaccines could lead to overall cost savings. Table 3 outlines the estimated overall health sector cost by roll-out scenario.
Table 3. Health care cost per vaccine in thousand USD
|
Vaccine |
Disease slower scenario |
Infection slower scenario |
Disease faster scenario |
Infection faster scenario |
|---|---|---|---|---|
|
AZ-like |
-$25,729 |
-$143,394 |
-$56,260 |
-$196,305 |
|
J&J-like |
$4,980 |
-$94,896 |
$4,180 |
-$123,893 |
|
Sinopharm -like |
$677,968 |
$589,242 |
$840,989 |
$737,200 |
|
Pfizer-like |
$282,395 |
$150,339 |
$331,566 |
$170,586 |
When measuring costs for every DALY, our modelling finds that viral vector vaccines are highly cost-effective, and in many scenarios, distributing them appears to be cost saving from a health sector perspective, meaning the ICER is negative (positive net health gains at lower overall cost relative to no vaccination). In other words, the savings from reduced hospitalisations and other medical treatments were usually greater than the cost of vaccination. Our modelling did not look at the wider economic benefits of COVID-19 vaccines, such reducing the need for lockdowns and increasing tourism, but these would further increase the return on investment. The faster scenario saw greater returns on investment than the slower one because the benefits of vaccination are greater the faster the population becomes vaccinated. However, there are constraints on how quickly vaccines can be rolled out.
The inactivated (Sinopharm-like) virus vaccine was both the most expensive and the least efficacious vaccine we modelled. Because of this, it likely did not make sense for Ethiopia to use this vaccine if the alternative COVID-19 vaccines modelled were available. Even if a vaccine similar to the one we modelled is the only vaccine available, our modelling suggests the Ethiopian government would likely pay between $2,300 and $5,200 per every DALY averted. This is between 2.6 and 6 times Ethiopia’s GDP per capita. Estimates by Ochalek et al. suggest that Ethiopia could avert between 6.3 and 20 times as many DALYs by spending this money elsewhere in its health system.
mRNA vaccines cost far more than either of the viral vector vaccines we modelled, but they are the most efficacious. The mRNA vaccine was modelled with an efficacy of 90 percent. The viral vector vaccines were modelled with 60 or 70 perecnt efficacy, because these vaccines have huge health benefits. Our model found that cost was a more important driver of cost-effectiveness than efficacy, however. Using the above inputs, our model found that an mRNA vaccine cost at least $600 more per DALY averted than the two viral vector vaccines. If there are no other vaccines available, it is plausibly good value for money to use an expensive mRNA vaccine with characteristics similar to those modelled in this study. However, this would only offer good value for money if officials believe the vaccine will greatly reduce transmission, or if it is targeted at vulnerable groups like the elderly. In most of the scenarios we examined, the mRNA vaccine would not have offered good value for money at the listed prices.
The results above are based on comparing a vaccine against no vaccine. When we instead compared the Pfizer-like vaccine against a viral vector vaccine, the Pfizer-like vaccine had even less value for money than the previous scenario, because large costs are paid for only a small increase in health relative to the viral vector vaccine. The ICER for this comparison rises to more than $2,300 per DALY averted in every model and over $6,000 when looking only at the disease model. A vaccine of this efficacy and price thus does not offer good value for money in Ethiopia when health officials have the option of buying viral vector vaccines. But it may if purchased at a lower price.
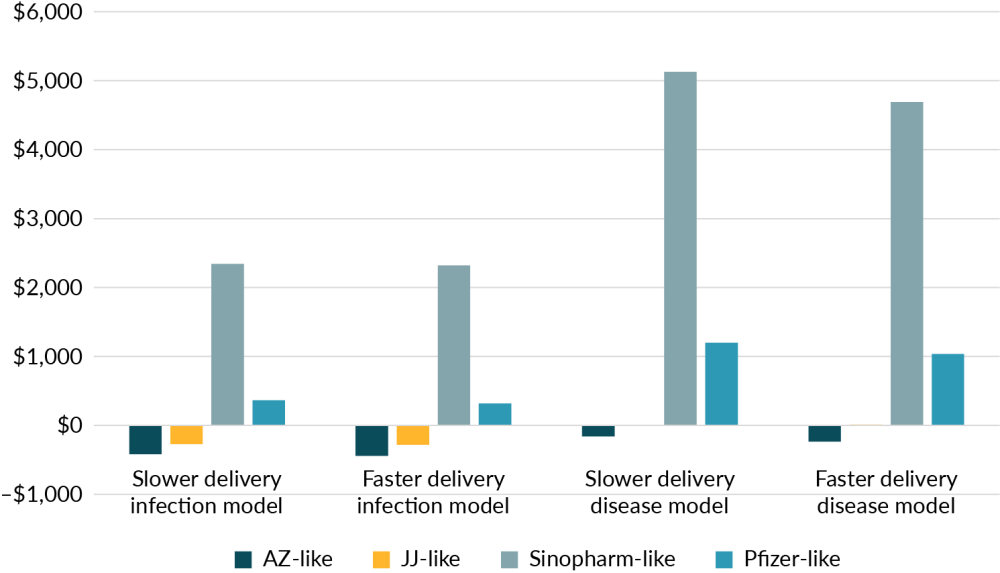
Figure 1 looks at the health system cost of averting a disability adjusted life year from each of the four vaccines. They are examined in both the faster and slower scenario and depending on whether the disease model or infection model was used. As shown, the two viral vector vaccines are far more cost effective than either the mRNA or inactivated virus vaccine, that were similar to Pfizer and Sinopharm respectively. These costs have been averaged by delivery method.
Figure 1. Cost per disability adjusted life year averted from using different vaccines in differing scenarios
Policy question 2: How should these vaccines be distributed?
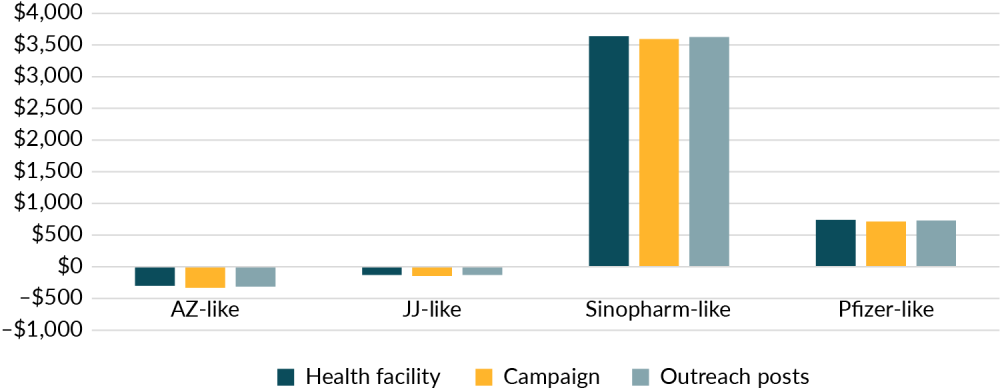
Our research suggests that the difference between the price of different vaccine delivery methods does not have a significant impact on the overall cost-effectiveness of the vaccination programme. For this reason, the government should focus on rollout mechanisms that can reach the most people or distribute vaccines fastest. Figure 2 shows the cost of averting a disability adjusted life year averaged across the faster and slower scenarios in both the disease and infection model, indicating that the delivery method has a much smaller impact on the benefits of a vaccine than the type of vaccine used.
Figure 2. The cost per disability adjusted life year does not vary significantly by delivery method
Policy question 3: What age groups should be targeted?
The vaccines are most efficacious at helping those with high-risk from COVID-19. Accordingly, vaccinating older people is far more cost-effective than vaccinating the young. It thus makes sense to prioritise older people first, and whilst not explicitly modelled, the same should be true for inoculating other high-risk groups. Our results suggest it is still likely cost effective to vaccinate younger people too, though only with viral vector vaccines similar to those produced by AstraZeneca and J&J which are less expensive than mRNA vaccines. If Ethiopia could procure mRNA vaccines at a much lower cost than used in this model, these would also be cost-effective in lower-risk groups.
Conclusion
The COVID-19 pandemic is fast moving, with new variants and vaccine supply fluctuations that are hard to predict. Policymakers face incredible challenges in protecting their citizens and we hope our analysis can help optimise future policy.
Our analysis shows that the benefits of vaccination are greatest when vaccines are administered widely and quickly; that vaccinating older and more vulnerable people first is important; and that the price of vaccines is a much stronger driver of cost effectiveness than efficacy. Policymakers in Ethiopia should prioritise lower-cost doses and, where possible, negotiate with pharmaceutical companies to secure an affordable price.
Acknowledgments
We would like to thank the organizations and professionals involved in this study. Our special thanks go to the maternal and child health directorate at the ministry of health; Yohannes Lakew and Melkamu Ayalew for their unreserved support; Africa CDC, especially Justice Nonvignon and Elias Asfaw, for their inputs into this project; Tom Drake at CGD for his support and for overseeing iDSI’s series of vaccine HTA projects.
Appendix. Average ICER per vaccine
This table averages each base case run of the model and shows the incremental cost per vaccine.
|
Scenario |
AZ - like |
JJ - like |
Sinopharm - like |
Pfizer - like |
|---|---|---|---|---|
|
Base case average |
-$313 |
-$134 |
$3,620 |
$729 |
|
Slower delivery infection model |
-$419 |
-$270 |
$2,340 |
$363 |
|
Faster delivery infection model |
-$442 |
-$281 |
$2,321 |
$319 |
|
Slower delivery disease model |
-$158 |
$4 |
$5,129 |
$1,199 |
|
Faster delivery disease model |
-$235 |
$12 |
$4,691 |
$1,036 |
|
Health facility |
-$300 |
-$129 |
$3,640 |
$741 |
|
Campaign |
-$330 |
-$144 |
$3,596 |
$715 |
|
Outreach posts |
-$311 |
-$129 |
$3,625 |
$732 |
Rights & Permissions
You may use and disseminate CGD’s publications under these conditions.