Recommended
Key takeaways
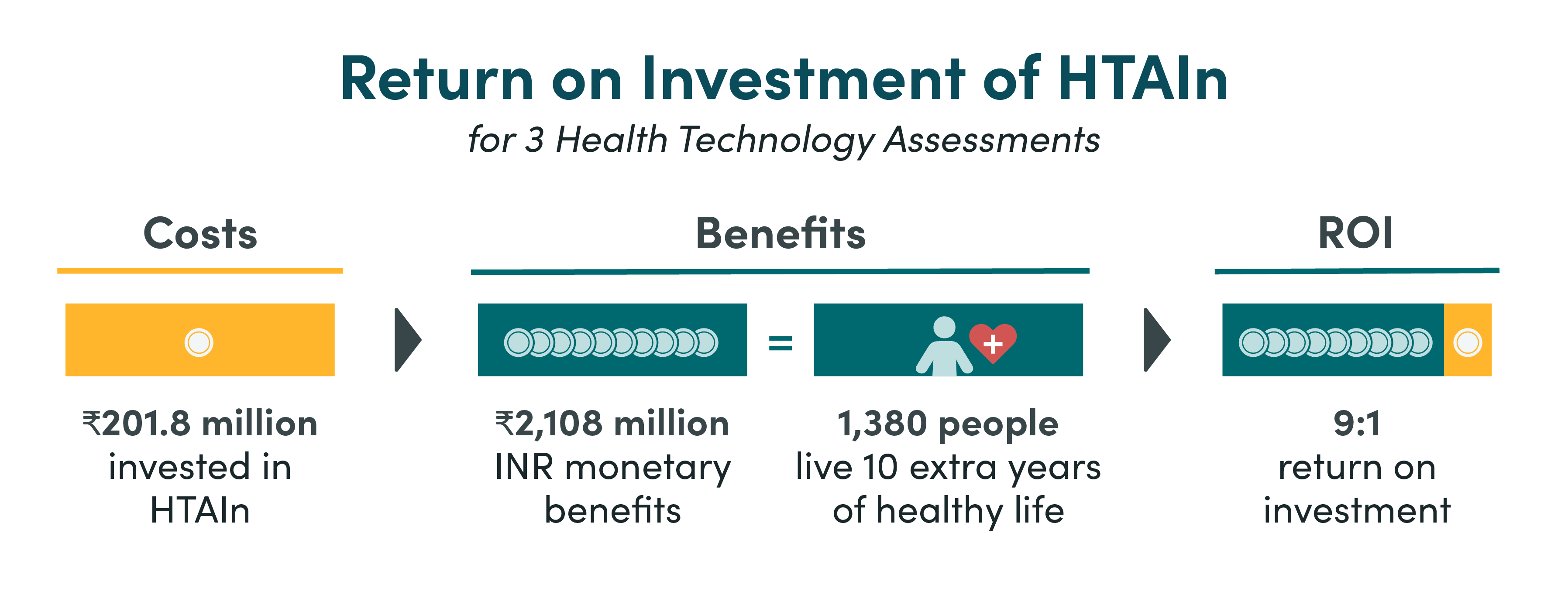
- We found that HTAIn has an overall realised return of 9:1 when comparing the total costs of Health Technology Assessment India (HTAIn) to the benefits from three Health Technology Assessments (HTAs)—safety engineered syringes, cervical cancer screening strategies and the Truenat diagnostic platform.
- Individual HTA returns ranged from 5:1 to 40:1.
- The realised net health benefits produced by these three HTAs is equivalent to over 1380 people living an extra 10 years of healthy life.
- The implementation level of HTA recommendations is critical to maximise the return on investment (ROI). We used conservative implementation estimates, but if we assume 100 percent implementation (and attribution) for these HTAs, the overall return of HTAIn could be as high as 71:1.
Background
Healthcare budgets around the world are under growing pressures, exacerbated by the COVID-19 pandemic, the military conflict in Ukraine, and subsequent economic, fiscal and debt crises. In these circumstances, it is more important than ever that governments institutionalise evidence-based priority setting processes, such as HTA to rigorously, systematically, and transparently allocate finite health resources based on evidence to maximise health benefit and support universal health coverage (UHC). Priority setting institutions that use HTA methods have an important role to play in supporting health systems to decide which health services and technologies to fund, to whom, and at what cost. However, since such institutions can divert resources away from other health priorities, it is important to understand the extent to which they are providing value for money. This evidence can help governments decide whether, and to what extent, to invest in HTA institutions.
The International Decision Support Initiative, a network of partners working with governments in health priority setting with a secretariat at the Center for Global Development, has supported the institutionalisation of HTA in India (HTAIn) since 2016. Established in 2017, HTAIn generates evidence to support central and state governments to make more transparent, inclusive, and evidence-based decisions related to healthcare spending, resource allocation and prioritisation, price negotiations, and regulation. As of 2021, HTAIn has completed more than 30 HTAs on a variety of topics. This evaluation aims to quantify the ROI achieved by HTAIn by extrapolating the ROI of three HTAs which had policy impact. This impact assessment is timely, as parliament considers a tabled HTA Bill.
Methods and assumptions
To estimate the ROI of HTAIn, we looked at the aggregate value of three HTAs, selected from a list of HTAs completed between 2017-2020. These HTAs are Safety Engineered Syringes (SES), various Cervical Cancer Screening strategies (CCS), and TrueNat rapid molecular test for diagnosis of infectious diseases. These HTAs were selected based on data availability, their range of interventions and target populations, and because their recommendations were implemented. Details and assumptions for each of the selected HTAs are presented in Table 1.
Table 1. Selected HTA details and assumptions
|
HTA |
Year HTA Completed |
Intervention Implemented |
Decision-Maker Who Used HTA |
Assumed Implementation Geography |
Estimated Implementation (%) |
|---|---|---|---|---|---|
|
SES |
2018 |
Safety-engineered syringes (SES) for therapeutic care |
Punjab State Government and National Pharmaceutical Pricing Authority, Government of India (GoI) |
Punjab State |
80% (Expert Opinion) |
|
Various CCS strategies |
2019 |
Three strategies for screening cervical cancer among women aged 30–65 years: visual inspection with acetic acid, Pap smear, and HPV DNA |
Non-Communicable Diseases (NCD) division, Punjab and NCD Division, Ministry of Health and Family Welfare, GoI |
All of India |
1.9% |
|
Truenat |
2019 |
Truenat for TB diagnosis |
Central Tuberculosis Division, GoI |
Andhra Pradesh |
82% |
To calculate the overall HTAIn ROI, we took the annual costs of HTAIn as its actual expenditure from 2019-20; and to calculate the ROI of each HTA, we averaged the annual cost of an HTA over 2018-2020. The ROI was then calculated by aggregating the (modelled) net health benefits (NHB) of the three HTAs and comparing these to the total costs. NHBs were expressed as net monetary benefits (NMB) using a 1 x Gross Domestic Product (GDP) threshold as recommended by HTAIn. We consider both health and monetary benefits to get a full picture of the return on investment. This means that while interventions may be cost-effective, they are not necessarily cost saving and do not represent a cash return. Please see the appendix for further method details.
Limitations
We assume full attribution of the benefits of implementation to the HTA recommendation. While true attribution is unlikely to be 100 percent, the counter-factual is not known i.e., what the level of implementation of each technology might have been without the HTA. This study is also limited given we are only calculating ROI based on one year (2019-20) of costs and benefits and using only a small sample of selected HTAs. While we tried to select a range of HTAs based on a variety of technologies and eligible populations, we were also constrained by data availability. Our analysis only captures the benefits of rolling out a technology that’s cost-effective in the HTA but there are likely to be many additional benefits from these HTAs not captured including better bargaining power on price negotiations, improved administrative systems or quality improvements. As such, we are likely to be conservative in illustrating the value of HTAIn.
Findings
The inferred ROI for HTAIn is large but with a wide range. The three HTAs assessed have a positive overall ROI of 9:1, with the ROI of each HTA ranging from 5:1 (SES, CCS) up to 40:1 (Truenat), as shown in Tables 2a and 2b. This shows that even when only considering three HTAs against all the costs of HTAIn, HTAIn still makes a strong return on their investment and presents good value for money. The realised net health benefits produced by three HTAs is equivalent to over 1380 people living an extra 10 years of healthy life.
Table 2a. ROI of HTAIn’s three studies
|
HTAs |
HTAIn Costs million (INR) |
Potential NMB (millions) |
Realised NMB (millions) |
Realised NHB |
ROI ratio Realised NMB |
ROI ratio potential NMB |
|---|---|---|---|---|---|---|
|
1. SES |
40.36 |
300 |
240 |
1573 |
5:1 |
6:1 |
|
2. CCS |
40.36 |
12,150 |
231 |
1514 |
5:1 |
300:1 |
|
3. TrueNat |
40.36 |
1,990 |
1638 |
10,742 |
40:1 |
48:1 |
Table 2b. Inferred ROI for HTAIn
|
|
HTAIn Costs million (INR) |
Potential NMB (millions) |
Realised NMB (millions) |
Realised NHB |
ROI ratio Realised NMB |
ROI ratio potential NMB |
|---|---|---|---|---|---|---|
|
ROI 3 HTAs v Annual cost HTAIn |
201.8 (Annual cost HTAIn) |
14,441 (3 HTAs) |
2,108 (3 HTAs) |
13,830 (3 HTAs) |
9:1
|
71:1
|
Figure 1. ROI of HTAIn
Degree of implementation is critical for ROI
If we calculate the ROI based on the potential benefits (assuming 100 percent implementation, rather than estimates of real implementation levels), then the overall ROI of HTAIn increases almost eight-fold to 71:1. The ROI of each HTA also increases. For example, current implementation of CCS at 1.9 percent leads to a realised ROI of 5:1. However, with 100 percent implementation this would increase significantly to 300:1. Implementation of HTA recommendations is therefore critical to optimising the ROI of HTAIn. It is only when decisions are implemented effectively, and patients receive the benefits, that India can secure the full impact of HTAIn.
Increasing the ROI of HTAIn
HTAIn has shown a good realised return on investment of 9:1. To further increase the return on investment, India can strategically scale up HTAIn and carry out careful selection of which topics to carry out HTAs on. For example, focusing on areas with ongoing uncertainty, large budget impact, and where the HTA recommendation will be adhered to and implemented.
HTA evidence uptake by public health sector ‘user’ departments must be supported to secure implementation and maximise HTA investment return. This requires reinforcing HTAIn’s mandate and role in health system governance and clarifying the responsibilities of stakeholders to implement HTA recommendations. It also means engaging stakeholders and decision-makers more deeply at the federal and state level to customise HTAIn’s approach to their policy requirements, in order to provide actionable recommendations that facilitate implementation.
Technical appendix available here.
Rights & Permissions
You may use and disseminate CGD’s publications under these conditions.